Novel concepts in male factor infertility: clinical and laboratory perspectives
- PMID: 27423664
- PMCID: PMC5065546
- DOI: 10.1007/s10815-016-0763-8
Novel concepts in male factor infertility: clinical and laboratory perspectives
Abstract
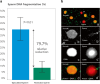
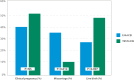
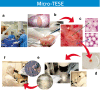
In recent years, the management of male factor infertility has undergone important changes with the introduction of novel concepts, advanced testing, and therapeutic interventions. This review highlights some of these changes and discusses their impact to routine clinical practice. First, we discuss the recent changes in the World Health Organization (WHO) laboratory methods and reference values for the examination of human semen. Second, we examine the role of sperm chromatin integrity tests in light of increasing evidence of the detrimental effect of sperm DNA fragmentation on reproductive outcomes. Third, we summarize the main findings of varicocele-related infertility and the outcomes of microsurgical varicocele repair to different case scenarios. Lastly, we critically discuss the current management of men with nonobstructive azoospermia seeking fertility and the new opportunities that emerged to help these men achieve biological fatherhood.
Keywords: Andrology; Male infertility; Microsurgery; Nonobstructive azoospermia; Semen analysis; Sperm DNA fragmentation; Varicocele.
Conflict of interest statement
The author declares that he has no conflicts of interest.
Figures




Similar articles
-
Specialized sperm function tests in varicocele and the future of andrology laboratory.Asian J Androl. 2016 Mar-Apr;18(2):205-12. doi: 10.4103/1008-682X.172642. Asian J Androl. 2016. PMID: 26780873 Free PMC article. Review.
-
Nomograms for predicting changes in semen parameters in infertile men after varicocele repair.Fertil Steril. 2014 Jul;102(1):68-74. doi: 10.1016/j.fertnstert.2014.03.046. Epub 2014 May 10. Fertil Steril. 2014. PMID: 24825425
-
Varicocele and nonobstructive azoospermia.Asian J Androl. 2025 May 1;27(3):355-360. doi: 10.4103/aja202444. Epub 2024 Aug 6. Asian J Androl. 2025. PMID: 39104262 Free PMC article. Review.
-
Microsurgical varicocele ligation: surgical methodology and associated outcomes.Fertil Steril. 2019 Mar;111(3):415-419. doi: 10.1016/j.fertnstert.2019.01.002. Fertil Steril. 2019. PMID: 30827515 Review.
-
Association of decreased spermatozoa omega-3 fatty acid levels and increased oxidative DNA damage with varicocele in infertile men: a case control study.Reprod Fertil Dev. 2016 Apr;28(5):648-54. doi: 10.1071/RD14276. Reprod Fertil Dev. 2016. PMID: 25405715
Cited by
-
From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology.Medicina (Kaunas). 2023 Oct 15;59(10):1835. doi: 10.3390/medicina59101835. Medicina (Kaunas). 2023. PMID: 37893553 Free PMC article. Review.
-
Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS) : Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR).J Endocrinol Invest. 2022 May;45(5):1085-1113. doi: 10.1007/s40618-022-01741-6. Epub 2022 Jan 24. J Endocrinol Invest. 2022. PMID: 35075609
-
Effect of varicocele repair on sperm DNA fragmentation: a review.Int Urol Nephrol. 2018 Apr;50(4):583-603. doi: 10.1007/s11255-018-1839-4. Epub 2018 Mar 14. Int Urol Nephrol. 2018. PMID: 29542060 Review.
-
Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis.Cell Death Dis. 2019 Jul 17;10(8):541. doi: 10.1038/s41419-019-1782-z. Cell Death Dis. 2019. PMID: 31316051 Free PMC article. Review.
-
Technical aspects of sperm DNA fragmentation testing, methods to select sperm with low DNA fragmentation, and usefulness of redox potential measurement in male infertility.Transl Androl Urol. 2017 Sep;6(Suppl 4):S636-S639. doi: 10.21037/tau.2017.05.24. Transl Androl Urol. 2017. PMID: 29082972 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

