Research Opportunities to Improve Neonatal Red Blood Cell Transfusion
- PMID: 27424006
- PMCID: PMC5026588
- DOI: 10.1016/j.tmrv.2016.06.005
Research Opportunities to Improve Neonatal Red Blood Cell Transfusion
Abstract
Red blood cell (RBC) transfusion is a common and lifesaving therapy for anemic neonates and infants, particularly among those born prematurely or undergoing surgery. However, evidence-based indications for when to administer RBCs and adverse effects of RBC transfusion on important outcomes including necrotizing enterocolitis, survival, and long-term neurodevelopmental impairment remain uncertain. In addition, blood-banking practices for preterm and term neonates and infants have been largely developed using studies from older children and adults. Use of and refinements in emerging technologies and advances in biomarker discovery and neonatal-specific RBC transfusion databases may allow clinicians to better define and tailor RBC transfusion needs and practices to individual neonates. Decreasing the need for RBC transfusion and developing neonatal-specific approaches in the preparation of donor RBCs have potential for reducing resource utilization and cost, improving outcomes, and assuring blood safety. Finally, large donor-recipient-linked cohort studies can provide data to better understand the balance of the risks and benefits of RBC transfusion in neonates. These studies may also guide the translation of new research into best practices that can rapidly be integrated into routine care. This review highlights key opportunities in transfusion medicine and neonatology for improving the preparation and transfusion of RBCs into neonates and infants. We focus on timely, currently addressable knowledge gaps that can increase the safety and efficacy of preterm and term neonatal and infant RBC transfusion practices.
Keywords: Anemia; Hemoglobin; Infant; Newborn; Prematurity, blood bank.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Ravi M. Patel and Erin K. Meyer have no potential conflicts of interest for this publication. John A. Widness serves on the scientific advisory board for HemoGenix Corporation.
Figures
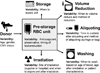
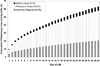

References
-
- Keir AK, Yang J, Harrison A, Pelausa E, Shah PS, Canadian Neonatal N. Temporal changes in blood product usage in preterm neonates born at less than 30 weeks' gestation in Canada. Transfusion. 2015;55:1340–1346. - PubMed
-
- Wesley MC, Yuki K, Daaboul DG, Dinardo JA. Blood utilization in neonates and infants undergoing cardiac surgery requiring cardiopulmonary bypass. World J Pediatr Congenit Heart Surg. 2011;2:382–392. - PubMed
-
- Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based medicine: how to practice and teach it. 4th. Edinburgh: Churchill Livingstone; 2004.
-
- Profit J, Soll RF. Neonatal networks: clinical research and quality improvement. Semin Fetal Neonatal Med. 2015;20:410–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

