Distinct functional domains of PNMA5 mediate protein-protein interaction, nuclear localization, and apoptosis signaling in human cancer cells
- PMID: 27424190
- PMCID: PMC11819373
- DOI: 10.1007/s00432-016-2205-5
Distinct functional domains of PNMA5 mediate protein-protein interaction, nuclear localization, and apoptosis signaling in human cancer cells
Abstract
Purpose: Members of paraneoplastic Ma (PNMA) family have been identified as onconeuronal antigens, which aberrant expressions in cancer cells of patients with paraneoplastic disorder (PND) are closely linked to manifestation of auto-immunity, neuro-degeneration, and cancer. The purpose of present study was to determine the role of PNMA5 and its functional relationship to MOAP-1 (PNMA4) in human cancer cells.
Methods: PNMA5 mutants were generated through deletion or site-directed mutagenesis and transiently expressed in human cancer cell lines to investigate their role in apoptosis, subcellular localization, and potential interaction with MOAP-1 through apoptosis assays, fluorescence microscopy, and co-immunoprecipitation studies, respectively.
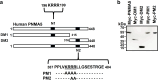
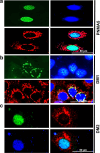
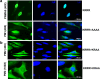
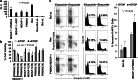
Results: Over-expressed human PNMA5 exhibited nuclear localization pattern in both MCF-7 and HeLa cells. Deletion mapping and mutagenesis studies showed that C-terminus of PNMA5 is responsible for nuclear localization, while the amino acid residues (391KRRR) within the C-terminus of PNMA5 are required for nuclear targeting. Deletion mapping and co-immunoprecipitation studies showed that PNMA5 interacts with MOAP-1 and N-terminal domain of PNMA5 is required for interaction with MOAP-1. Furthermore, co-expression of PNMA5 and MOAP-1 in MCF-7 cells significantly enhanced chemo-sensitivity of MCF-7 to Etoposide treatment, indicating that PNMA5 and MOAP-1 interact synergistically to promote apoptotic signaling in MCF-7 cells.
Conclusions: Our results show that PNMA5 promotes apoptosis signaling in HeLa and MCF-7 cells and interacts synergistically with MOAP-1 through its N-terminal domain to promote apoptosis and chemo-sensitivity in human cancer cells. The C-terminal domain of PNMA5 is required for nuclear localization; however, both N-and C-terminal domains of PNMA5 appear to be required for pro-apoptotic function.
Keywords: Apoptosis; Chemo-sensitivity; Functional domains; MOAP-1; PNMA5; Paraneoplastic Antigens.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Tricistronic expression of MOAP-1, Bax and RASSF1A in cancer cells enhances chemo-sensitization that requires BH3L domain of MOAP-1.J Cancer Res Clin Oncol. 2020 Jul;146(7):1751-1764. doi: 10.1007/s00432-020-03231-9. Epub 2020 May 6. J Cancer Res Clin Oncol. 2020. PMID: 32377840 Free PMC article.
-
PNMA2 mediates heterodimeric interactions and antagonizes chemo-sensitizing activities mediated by members of PNMA family.Biochem Biophys Res Commun. 2016 Apr 22;473(1):224-229. doi: 10.1016/j.bbrc.2016.03.083. Epub 2016 Mar 19. Biochem Biophys Res Commun. 2016. PMID: 27003254
-
Autographa californica multiple nucleopolyhedrovirus Ac51 interacts with Ac66 and facilitates its nuclear localization to promote the nuclear egress of nucleocapsids.J Virol. 2025 Jun 17;99(6):e0196924. doi: 10.1128/jvi.01969-24. Epub 2025 May 28. J Virol. 2025. PMID: 40434106 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
Cited by
-
PNMA5 knockdown suppresses the proliferation, invasion, and metastasis of epithelial ovarian cancer cells.Transl Cancer Res. 2020 May;9(5):3691-3702. doi: 10.21037/tcr-20-2013. Transl Cancer Res. 2020. PMID: 35117731 Free PMC article.
-
PNMA5 Promotes Bone Metastasis of Non-small-Cell Lung Cancer as a Target of BMP2 Signaling.Front Cell Dev Biol. 2021 May 31;9:678931. doi: 10.3389/fcell.2021.678931. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34136487 Free PMC article.
-
Revealing the Roles of MOAP1 in Diseases: A Review.Cells. 2022 Mar 4;11(5):889. doi: 10.3390/cells11050889. Cells. 2022. PMID: 35269511 Free PMC article. Review.
-
Transcriptome analysis of the adult human Klinefelter testis and cellularity-matched controls reveals disturbed differentiation of Sertoli- and Leydig cells.Cell Death Dis. 2018 May 22;9(6):586. doi: 10.1038/s41419-018-0671-1. Cell Death Dis. 2018. PMID: 29789566 Free PMC article.
-
Human paraneoplastic antigen Ma2 (PNMA2) forms icosahedral capsids that can be engineered for mRNA delivery.Proc Natl Acad Sci U S A. 2024 Mar 12;121(11):e2307812120. doi: 10.1073/pnas.2307812120. Epub 2024 Mar 4. Proc Natl Acad Sci U S A. 2024. PMID: 38437549 Free PMC article.
References
-
- Allen NPC, Donninger H, Vos MD et al (2007) RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26:6203–6211. doi:10.1038/sj.onc.1210440 - PubMed
-
- Archer HA, Panopoulou A, Bhatt N et al (2014) Mesothelioma and anti-Ma paraneoplastic syndrome; heterogeneity in immunogenic tumours increases. Pract Neurol 14:33–35. doi:10.1136/practneurol-2013-000520 - PubMed
-
- Baksh S, Tommasi S, Fenton S et al (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to bax conformational change and cell death. Mol Cell 18:637–650. doi:10.1016/j.molcel.2005.05.010 - PubMed
-
- Braik T, Evans AT, Telfer M, McDunn S (2010) Paraneoplastic neurological syndromes: unusual presentations of cancer. A practical review. Am J Med Sci 340:301–308. doi:10.1097/MAJ.0b013e3181d9bb3b - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

