Distinct functional domains of PNMA5 mediate protein-protein interaction, nuclear localization, and apoptosis signaling in human cancer cells
- PMID: 27424190
- PMCID: PMC11819373
- DOI: 10.1007/s00432-016-2205-5
Distinct functional domains of PNMA5 mediate protein-protein interaction, nuclear localization, and apoptosis signaling in human cancer cells
Abstract
Purpose: Members of paraneoplastic Ma (PNMA) family have been identified as onconeuronal antigens, which aberrant expressions in cancer cells of patients with paraneoplastic disorder (PND) are closely linked to manifestation of auto-immunity, neuro-degeneration, and cancer. The purpose of present study was to determine the role of PNMA5 and its functional relationship to MOAP-1 (PNMA4) in human cancer cells.
Methods: PNMA5 mutants were generated through deletion or site-directed mutagenesis and transiently expressed in human cancer cell lines to investigate their role in apoptosis, subcellular localization, and potential interaction with MOAP-1 through apoptosis assays, fluorescence microscopy, and co-immunoprecipitation studies, respectively.
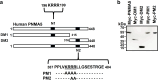
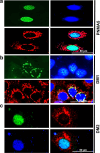
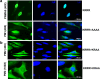
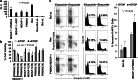
Results: Over-expressed human PNMA5 exhibited nuclear localization pattern in both MCF-7 and HeLa cells. Deletion mapping and mutagenesis studies showed that C-terminus of PNMA5 is responsible for nuclear localization, while the amino acid residues (391KRRR) within the C-terminus of PNMA5 are required for nuclear targeting. Deletion mapping and co-immunoprecipitation studies showed that PNMA5 interacts with MOAP-1 and N-terminal domain of PNMA5 is required for interaction with MOAP-1. Furthermore, co-expression of PNMA5 and MOAP-1 in MCF-7 cells significantly enhanced chemo-sensitivity of MCF-7 to Etoposide treatment, indicating that PNMA5 and MOAP-1 interact synergistically to promote apoptotic signaling in MCF-7 cells.
Conclusions: Our results show that PNMA5 promotes apoptosis signaling in HeLa and MCF-7 cells and interacts synergistically with MOAP-1 through its N-terminal domain to promote apoptosis and chemo-sensitivity in human cancer cells. The C-terminal domain of PNMA5 is required for nuclear localization; however, both N-and C-terminal domains of PNMA5 appear to be required for pro-apoptotic function.
Keywords: Apoptosis; Chemo-sensitivity; Functional domains; MOAP-1; PNMA5; Paraneoplastic Antigens.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Allen NPC, Donninger H, Vos MD et al (2007) RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26:6203–6211. doi:10.1038/sj.onc.1210440 - PubMed
-
- Archer HA, Panopoulou A, Bhatt N et al (2014) Mesothelioma and anti-Ma paraneoplastic syndrome; heterogeneity in immunogenic tumours increases. Pract Neurol 14:33–35. doi:10.1136/practneurol-2013-000520 - PubMed
-
- Baksh S, Tommasi S, Fenton S et al (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to bax conformational change and cell death. Mol Cell 18:637–650. doi:10.1016/j.molcel.2005.05.010 - PubMed
-
- Braik T, Evans AT, Telfer M, McDunn S (2010) Paraneoplastic neurological syndromes: unusual presentations of cancer. A practical review. Am J Med Sci 340:301–308. doi:10.1097/MAJ.0b013e3181d9bb3b - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

