Cabazitaxel overcomes cisplatin resistance in germ cell tumour cells
- PMID: 27424191
- PMCID: PMC11818977
- DOI: 10.1007/s00432-016-2204-6
Cabazitaxel overcomes cisplatin resistance in germ cell tumour cells
Abstract
Background: Cisplatin-based chemotherapy is highly effective in metastasized germ cell tumours (GCT). However, 10-30 % of patients develop resistance to cisplatin, requiring salvage therapy. We investigated the in vitro activity of paclitaxel and the novel taxane cabazitaxel in cisplatin-sensitive and -resistant GCT cell lines.
Methods: In vitro activity of paclitaxel and cabazitaxel was determined by proliferation assays, and mode of action of cabazitaxel was assessed by western blotting and two screening approaches, i.e. whole proteome analysis and a human apoptosis array.
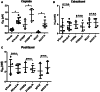
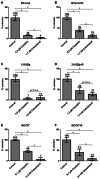
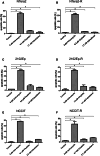
Results: Activity of paclitaxel and cabazitaxel was not affected by cisplatin resistance, suggesting that there is no cross-resistance between these agents in vitro. Cabazitaxel treatment showed a strong inhibitory effect on colony formation capacity. Cabazitaxel induced classical apoptosis in all cell lines, reflected by cleavage of PARP and caspase 3, without inducing specific changes in the cell cycle distribution. Using the proteomic and human apoptosis array screening approaches, differential regulation of several proteins, including members of the bcl-2 family, was found, giving first insights into the mode of action of cabazitaxel in GCT.
Conclusion: Cabazitaxel shows promising in vitro activity in GCT cells, independent of levels of cisplatin resistance.
Keywords: Apoptosis; Cabazitaxel; Cisplatin resistance; Germ cell tumour.
Conflict of interest statement
Mirjam Gerwing declares that she has no conflict of interest. Christine Jacobsen declares that she has no conflict of interest. Sergey Dyshlovoy declares that he has no conflict of interest. Jessica Hauschild declares that she has no conflict of interest. Tina Rohlfing declares that she has no conflict of interest. Christoph Oing declares that he has no conflict of interest. Simone Venz declares that she has no conflict of interest. Jan Oldenburg declares that he has no conflict of interest. Karin Oechsle declares that she has no conflict of interest. Carsten Bokemeyer declares that he has no conflict of interest. Gunhild von Amsberg declares that she has no conflict of interest. Friedemann Honecker declares that he has no conflict of interest.
Figures




Similar articles
-
Treatment of refractory germ-cell tumours with single-agent cabazitaxel: a German Testicular Cancer Study Group case series.J Cancer Res Clin Oncol. 2020 Feb;146(2):449-455. doi: 10.1007/s00432-019-03071-2. Epub 2019 Dec 14. J Cancer Res Clin Oncol. 2020. PMID: 31838576 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Refractory testicular germ cell tumors are highly sensitive to the targeting of polycomb pathway demethylases KDM6A and KDM6B.Cell Commun Signal. 2024 Oct 31;22(1):528. doi: 10.1186/s12964-024-01912-3. Cell Commun Signal. 2024. PMID: 39482699 Free PMC article.
-
Everolimus in patients with multiply relapsed or cisplatin refractory germ cell tumors: results of a phase II, single-arm, open-label multicenter trial (RADIT) of the German Testicular Cancer Study Group.J Cancer Res Clin Oncol. 2019 Mar;145(3):717-723. doi: 10.1007/s00432-018-2752-z. Epub 2018 Sep 19. J Cancer Res Clin Oncol. 2019. PMID: 30232558 Free PMC article. Clinical Trial.
-
A systematic overview of chemotherapy effects in ovarian cancer.Acta Oncol. 2001;40(2-3):340-60. doi: 10.1080/02841860151116420. Acta Oncol. 2001. PMID: 11441940
Cited by
-
Cabazitaxel as a promising therapy for cisplatin-resistant bladder cancer: a preliminary study.Med Oncol. 2024 Aug 6;41(9):219. doi: 10.1007/s12032-024-02461-y. Med Oncol. 2024. PMID: 39105986
-
Carbon monoxide: An emerging therapy for acute kidney injury.Med Res Rev. 2020 Jul;40(4):1147-1177. doi: 10.1002/med.21650. Epub 2019 Dec 9. Med Res Rev. 2020. PMID: 31820474 Free PMC article. Review.
-
Treatment of refractory germ-cell tumours with single-agent cabazitaxel: a German Testicular Cancer Study Group case series.J Cancer Res Clin Oncol. 2020 Feb;146(2):449-455. doi: 10.1007/s00432-019-03071-2. Epub 2019 Dec 14. J Cancer Res Clin Oncol. 2020. PMID: 31838576 Free PMC article.
-
Cisplatin‑resistant germ cell tumor models: An exploration of the epithelial‑mesenchymal transition regulator SLUG.Mol Med Rep. 2024 Dec;30(6):228. doi: 10.3892/mmr.2024.13352. Epub 2024 Oct 11. Mol Med Rep. 2024. PMID: 39392037 Free PMC article.
-
Epigenetic Remodeling through Downregulation of Polycomb Repressive Complex 2 Mediates Chemotherapy Resistance in Testicular Germ Cell Tumors.Cancers (Basel). 2019 Jun 8;11(6):796. doi: 10.3390/cancers11060796. Cancers (Basel). 2019. PMID: 31181810 Free PMC article.
References
-
- Aaronson RM, Graven KK, Tucci M, McDonald RJ, Farber HW (1995) Non-neuronal enolase is an endothelial hypoxic stress protein. J Biol Chem 270(46):27752–27757 - PubMed
-
- Andrews PW (1984) Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 103(2):285–293 - PubMed
-
- Azarenko O, Smiyun G, Mah J, Wilson L, Jordan MA (2014) Antiproliferative mechanism of action of the novel Taxane Cabazitaxel as compared with the parent compound Docetaxel in MCF7 breast cancer cells. Mol Cancer Ther 13(8):2092–2103 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

