Role of lipids in the metabolism and activation of immune cells
- PMID: 27424223
- PMCID: PMC5694687
- DOI: 10.1016/j.jnutbio.2015.11.002
Role of lipids in the metabolism and activation of immune cells
Abstract
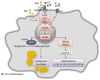
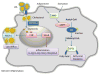
Immune cell plasticity has extensive implications in the pathogenesis and resolution of metabolic disorders, cancers, autoimmune diseases and chronic inflammatory disorders. Over the past decade, nutritional status has been discovered to influence the immune response. In metabolic disorders such as obesity, immune cells interact with various classes of lipids, which are capable of controlling the plasticity of macrophages and T lymphocytes. The purpose of this review is to discuss lipids and their impact on innate and adaptive immune responses, focusing on two areas: (1) the impact of altering lipid metabolism on immune cell activation, differentiation and function and (2) the mechanism by which lipids such as cholesterol and fatty acids regulate immune cell plasticity.
Keywords: Fatty acids; Inflammation; Macrophages; Oxysterols; T lymphocytes.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Adapted Immune Responses of Myeloid-Derived Cells in Fatty Liver Disease.Front Immunol. 2018 Oct 18;9:2418. doi: 10.3389/fimmu.2018.02418. eCollection 2018. Front Immunol. 2018. PMID: 30405618 Free PMC article. Review.
-
Innate Immune Signaling in Nonalcoholic Fatty Liver Disease and Cardiovascular Diseases.Annu Rev Pathol. 2019 Jan 24;14:153-184. doi: 10.1146/annurev-pathmechdis-012418-013003. Epub 2018 Sep 19. Annu Rev Pathol. 2019. PMID: 30230967 Review.
-
Obesity: does it modulate infectious disease and immunity?Prog Clin Biol Res. 1981;67:327-37. Prog Clin Biol Res. 1981. PMID: 7029562 Review.
-
Emerging Molecular Targets for Treatment of Nonalcoholic Fatty Liver Disease.Trends Endocrinol Metab. 2019 Dec;30(12):903-914. doi: 10.1016/j.tem.2019.08.006. Epub 2019 Oct 6. Trends Endocrinol Metab. 2019. PMID: 31597607 Review.
-
Macrophages and lipid metabolism.Cell Immunol. 2018 Aug;330:27-42. doi: 10.1016/j.cellimm.2018.01.020. Epub 2018 Feb 2. Cell Immunol. 2018. PMID: 29429624 Free PMC article. Review.
Cited by
-
Characterizing influence of rCHOP treatment on diffuse large B-cell lymphoma microenvironment through in vitro microfluidic spheroid model.Cell Death Dis. 2024 Jan 9;15(1):18. doi: 10.1038/s41419-023-06299-6. Cell Death Dis. 2024. PMID: 38195589 Free PMC article.
-
Signs of Deregulated Gene Expression Are Present in Both CD14+ and CD14- PBMC From Non-Obese Men With Family History of T2DM.Front Endocrinol (Lausanne). 2021 Feb 15;11:582732. doi: 10.3389/fendo.2020.582732. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33658980 Free PMC article.
-
Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths?Evol Med Public Health. 2016 Jan;2016(1):338-357. doi: 10.1093/emph/eow028. Epub 2016 Sep 25. Evol Med Public Health. 2016. PMID: 27666719 Free PMC article. Review.
-
The Immunometabolic Atlas: A tool for design and interpretation of metabolomics studies in immunology.PLoS One. 2022 May 12;17(5):e0268408. doi: 10.1371/journal.pone.0268408. eCollection 2022. PLoS One. 2022. PMID: 35550647 Free PMC article.
-
Samsum ant venom modulates the immune response and redox status at the acute toxic dose in vivo.J Venom Anim Toxins Incl Trop Dis. 2019 Dec 2;25:e20190020. doi: 10.1590/1678-9199-JVATITD-2019-0020. eCollection 2019. J Venom Anim Toxins Incl Trop Dis. 2019. PMID: 31839800 Free PMC article.
References
-
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

