Comparison of the ligand binding site of CYP2C8 with CYP26A1 and CYP26B1: a structural basis for the identification of new inhibitors of the retinoic acid hydroxylases
- PMID: 27424662
- PMCID: PMC6628712
- DOI: 10.1080/14756366.2016.1193734
Comparison of the ligand binding site of CYP2C8 with CYP26A1 and CYP26B1: a structural basis for the identification of new inhibitors of the retinoic acid hydroxylases
Abstract
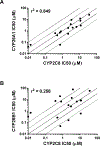
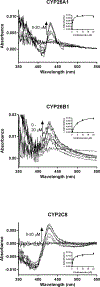
The CYP26s are responsible for metabolizing retinoic acid and play an important role in maintaining homeostatic levels of retinoic acid. Given the ability of CYP2C8 to metabolize retinoic acid, we evaluated the potential for CYP2C8 inhibitors to also inhibit CYP26. In vitro assays were used to evaluate the inhibition potencies of CYP2C8 inhibitors against CYP26A1 and CYP26B1. Using tazarotenic acid as a substrate for CYP26, IC50 values for 17 inhibitors of CYP2C8 were determined for CYP26A1 and CYP26B1, ranging from ∼20 nM to 100 μM, with a positive correlation observed between IC50s for CYP2C8 and CYP26A1. An evaluation of IC50's versus in vivo Cmax values suggests that inhibitors such as clotrimazole or fluconazole may interact with CYP26 at clinically relevant concentrations and may alter levels of retinoic acid. These findings provide insight into drug interactions resulting in elevated retinoic acid concentrations and expand upon the pharmacophore of CYP26 inhibition.
Keywords: CYP26; CYP2C8; homology model; retinoic acid; tazarotenic acid.
Figures





Similar articles
-
Identification of Tazarotenic Acid as the First Xenobiotic Substrate of Human Retinoic Acid Hydroxylase CYP26A1 and CYP26B1.J Pharmacol Exp Ther. 2016 May;357(2):281-92. doi: 10.1124/jpet.116.232637. Epub 2016 Mar 2. J Pharmacol Exp Ther. 2016. PMID: 26937021 Free PMC article.
-
Molecular recognition of CYP26A1 binding pockets and structure-activity relationship studies for design of potent and selective retinoic acid metabolism blocking agents.J Mol Graph Model. 2015 Mar;56:10-9. doi: 10.1016/j.jmgm.2014.11.014. Epub 2014 Dec 8. J Mol Graph Model. 2015. PMID: 25541526
-
Inhibition of the all-trans Retinoic Acid (atRA) Hydroxylases CYP26A1 and CYP26B1 Results in Dynamic, Tissue-Specific Changes in Endogenous atRA Signaling.Drug Metab Dispos. 2017 Jul;45(7):846-854. doi: 10.1124/dmd.117.075341. Epub 2017 Apr 26. Drug Metab Dispos. 2017. PMID: 28446509 Free PMC article.
-
Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics.Curr Top Med Chem. 2013;13(12):1402-28. doi: 10.2174/1568026611313120004. Curr Top Med Chem. 2013. PMID: 23688132 Free PMC article. Review.
-
Cytochrome P450s in the regulation of cellular retinoic acid metabolism.Annu Rev Nutr. 2011 Aug 21;31:65-87. doi: 10.1146/annurev-nutr-072610-145127. Annu Rev Nutr. 2011. PMID: 21529158 Free PMC article. Review.
Cited by
-
Computational model for fetal skeletal defects potentially linked to disruption of retinoic acid signaling.Front Pharmacol. 2022 Sep 6;13:971296. doi: 10.3389/fphar.2022.971296. eCollection 2022. Front Pharmacol. 2022. PMID: 36172177 Free PMC article.
-
Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases.Pharmacol Ther. 2019 Dec;204:107400. doi: 10.1016/j.pharmthera.2019.107400. Epub 2019 Aug 13. Pharmacol Ther. 2019. PMID: 31419517 Free PMC article. Review.
-
An Insight into the Metabolism of 2,5-Disubstituted Monotetrazole Bearing Bisphenol Structures: Emerging Bisphenol A Structural Congeners.Molecules. 2023 Feb 2;28(3):1465. doi: 10.3390/molecules28031465. Molecules. 2023. PMID: 36771130 Free PMC article.
-
Identifying candidate reference chemicals for in vitro testing of the retinoid pathway for predictive developmental toxicity.ALTEX. 2023;40(2):217–236. doi: 10.14573/altex.2202231. Epub 2022 Jun 23. ALTEX. 2023. PMID: 35796328 Free PMC article.
-
Divergent Roles of CYP26B1 and Endogenous Retinoic Acid in Mouse Fetal Gonads.Biomolecules. 2019 Sep 26;9(10):536. doi: 10.3390/biom9100536. Biomolecules. 2019. PMID: 31561560 Free PMC article.
References
-
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annual review of nutrition. 2002;22:347–81. - PubMed
-
- Lotan R Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochimica et biophysica acta. 1980;605(1):33–91. - PubMed
-
- Maden M Retinoid signalling in the development of the central nervous system. Nature reviews Neuroscience. 2002;3(11):843–53. - PubMed
-
- McCaffery P, Drager UC. Regulation of retinoic acid signaling in the embryonic nervous system: a master differentiation factor. Cytokine & growth factor reviews. 2000;11(3):233–49. - PubMed
-
- Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiological reviews. 2000;80(3):1021–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
