Ectopic miR-125a Expression Induces Long-Term Repopulating Stem Cell Capacity in Mouse and Human Hematopoietic Progenitors
- PMID: 27424784
- PMCID: PMC5500905
- DOI: 10.1016/j.stem.2016.06.008
Ectopic miR-125a Expression Induces Long-Term Repopulating Stem Cell Capacity in Mouse and Human Hematopoietic Progenitors
Abstract
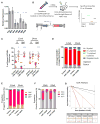
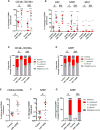
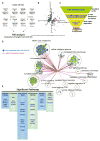
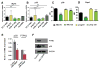
Umbilical cord blood (CB) is a convenient and broadly used source of hematopoietic stem cells (HSCs) for allogeneic stem cell transplantation. However, limiting numbers of HSCs remain a major constraint for its clinical application. Although one feasible option would be to expand HSCs to improve therapeutic outcome, available protocols and the molecular mechanisms governing the self-renewal of HSCs are unclear. Here, we show that ectopic expression of a single microRNA (miRNA), miR-125a, in purified murine and human multipotent progenitors (MPPs) resulted in increased self-renewal and robust long-term multi-lineage repopulation in transplanted recipient mice. Using quantitative proteomics and western blot analysis, we identified a restricted set of miR-125a targets involved in conferring long-term repopulating capacity to MPPs in humans and mice. Our findings offer the innovative potential to use MPPs with enhanced self-renewal activity to augment limited sources of HSCs to improve clinical protocols.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Persistent expression of microRNA-125a targets is required to induce murine hematopoietic stem cell repopulating activity.Exp Hematol. 2021 Feb;94:47-59.e5. doi: 10.1016/j.exphem.2020.12.002. Epub 2020 Dec 15. Exp Hematol. 2021. PMID: 33333212
-
Ascorbate deficiency increases quiescence and self-renewal in hematopoietic stem cells and multipotent progenitors.Blood. 2025 Jan 2;145(1):114-126. doi: 10.1182/blood.2024024769. Blood. 2025. PMID: 39437548 Free PMC article.
-
The frequency of multipotent CD133(+)CD45RA(-)CD34(+) hematopoietic stem cells is not increased in fetal liver compared with adult stem cell sources.Exp Hematol. 2016 Jun;44(6):502-7. doi: 10.1016/j.exphem.2016.02.011. Epub 2016 Mar 22. Exp Hematol. 2016. PMID: 27016273 Free PMC article.
-
[Updated human hematopoietic stem cell findings: purification of human hematopoietic stem cells and elucidation of their hierarchy].Rinsho Ketsueki. 2018;59(10):1861-1871. doi: 10.11406/rinketsu.59.1861. Rinsho Ketsueki. 2018. PMID: 30305486 Review. Japanese.
-
Micro-RNAs: A safety net to protect hematopoietic stem cell self-renewal.Wiley Interdiscip Rev RNA. 2022 May;13(3):e1693. doi: 10.1002/wrna.1693. Epub 2021 Sep 16. Wiley Interdiscip Rev RNA. 2022. PMID: 34532984 Free PMC article. Review.
Cited by
-
Hoxb5 reprogrammes murine multipotent blood progenitors into haematopoietic stem cell-like cells.Cell Prolif. 2022 Jun;55(6):e13235. doi: 10.1111/cpr.13235. Epub 2022 May 17. Cell Prolif. 2022. PMID: 35582777 Free PMC article.
-
Loss of ING4 enhances hematopoietic regeneration in multipotent progenitor cells.PLoS One. 2025 Feb 14;20(2):e0316256. doi: 10.1371/journal.pone.0316256. eCollection 2025. PLoS One. 2025. PMID: 39951458 Free PMC article.
-
NUP98-HOXA10hd fusion protein sustains multi-lineage haematopoiesis of lineage-committed progenitors in transplant setting.Cell Prolif. 2020 Sep;53(9):e12885. doi: 10.1111/cpr.12885. Epub 2020 Jul 29. Cell Prolif. 2020. PMID: 32725842 Free PMC article.
-
Targeting JNK pathway promotes human hematopoietic stem cell expansion.Cell Discov. 2019 Jan 8;5:2. doi: 10.1038/s41421-018-0072-8. eCollection 2019. Cell Discov. 2019. PMID: 30622738 Free PMC article.
-
Identification of the global miR-130a targetome reveals a role for TBL1XR1 in hematopoietic stem cell self-renewal and t(8;21) AML.Cell Rep. 2022 Mar 8;38(10):110481. doi: 10.1016/j.celrep.2022.110481. Cell Rep. 2022. PMID: 35263585 Free PMC article.
References
-
- Aljurf M, Rizzo JD, Mohty M, Hussain F, Madrigal A, Pasquini MC, Passweg J, Chaudhri N, Ghavamzadeh A, Solh HE, et al. Challenges and opportunities for HSCT outcome registries: perspective from international HSCT registries experts. Bone Marrow Transplant. 2014;49:1016–1021. - PubMed
-
- Balmain A, Harris CC. Carcinogenesis in mouse and human cells: parallels and paradoxes. Carcinogenesis. 2000;21:371–377. - PubMed
-
- Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

