Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma
- PMID: 27424807
- PMCID: PMC5374862
- DOI: 10.1016/j.ccell.2016.06.003
Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma
Abstract
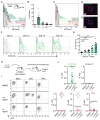
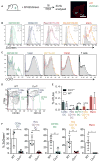
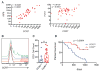
Intratumoral dendritic cells (DC) bearing CD103 in mice or CD141 in humans drive intratumoral CD8(+) T cell activation. Using multiple strategies, we identified a critical role for these DC in trafficking tumor antigen to lymph nodes (LN), resulting in both direct CD8(+) T cell stimulation and antigen hand-off to resident myeloid cells. These effects all required CCR7. Live imaging demonstrated direct presentation to T cells in LN, and CCR7 loss specifically in these cells resulted in defective LN T cell priming and increased tumor outgrowth. CCR7 expression levels in human tumors correlate with signatures of CD141(+) DC, intratumoral T cells, and better clinical outcomes. This work identifies an ongoing pathway to T cell priming, which should be harnessed for tumor therapies.
Keywords: 2-photon imaging; anti-tumor T cell priming; antigen presentation; antigen trafficking; dendritic cells; draining lymph node; melanoma; tumor; tumor immune response.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. - PubMed
-
- Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. - PubMed
-
- Bogunovic D, O’Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–20434. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

