Antiretroviral Therapy for the Prevention of HIV-1 Transmission
- PMID: 27424812
- PMCID: PMC5049503
- DOI: 10.1056/NEJMoa1600693
Antiretroviral Therapy for the Prevention of HIV-1 Transmission
Abstract
Background: An interim analysis of data from the HIV Prevention Trials Network (HPTN) 052 trial showed that antiretroviral therapy (ART) prevented more than 96% of genetically linked infections caused by human immunodeficiency virus type 1 (HIV-1) in serodiscordant couples. ART was then offered to all patients with HIV-1 infection (index participants). The study included more than 5 years of follow-up to assess the durability of such therapy for the prevention of HIV-1 transmission.
Methods: We randomly assigned 1763 index participants to receive either early or delayed ART. In the early-ART group, 886 participants started therapy at enrollment (CD4+ count, 350 to 550 cells per cubic millimeter). In the delayed-ART group, 877 participants started therapy after two consecutive CD4+ counts fell below 250 cells per cubic millimeter or if an illness indicative of the acquired immunodeficiency syndrome (i.e., an AIDS-defining illness) developed. The primary study end point was the diagnosis of genetically linked HIV-1 infection in the previously HIV-1-negative partner in an intention-to-treat analysis.
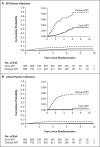
Results: Index participants were followed for 10,031 person-years; partners were followed for 8509 person-years. Among partners, 78 HIV-1 infections were observed during the trial (annual incidence, 0.9%; 95% confidence interval [CI], 0.7 to 1.1). Viral-linkage status was determined for 72 (92%) of the partner infections. Of these infections, 46 were linked (3 in the early-ART group and 43 in the delayed-ART group; incidence, 0.5%; 95% CI, 0.4 to 0.7) and 26 were unlinked (14 in the early-ART group and 12 in the delayed-ART group; incidence, 0.3%; 95% CI, 0.2 to 0.4). Early ART was associated with a 93% lower risk of linked partner infection than was delayed ART (hazard ratio, 0.07; 95% CI, 0.02 to 0.22). No linked infections were observed when HIV-1 infection was stably suppressed by ART in the index participant.
Conclusions: The early initiation of ART led to a sustained decrease in genetically linked HIV-1 infections in sexual partners. (Funded by the National Institute of Allergy and Infectious Diseases; HPTN 052 ClinicalTrials.gov number, NCT00074581 .).
Figures

Comment in
-
Early initiation of antiretroviral therapy prevents HIV transmission to seronegative sexual partners.Evid Based Med. 2017 Mar;22(1):29. doi: 10.1136/ebmed-2016-110576. Epub 2016 Nov 16. Evid Based Med. 2017. PMID: 27852628 No abstract available.
References
-
- Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–9. - PubMed
-
- People living with HIV. UNAIDS; 2014. ( http://aidsinfo.unaids.org/#)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1-AI068619/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01-AI068619/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI068613/AI/NIAID NIH HHS/United States
- UM1-AI068613/AI/NIAID NIH HHS/United States
- U01-AI068617/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01-AI068613/AI/NIAID NIH HHS/United States
- U01 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- U01 AI068617/AI/NIAID NIH HHS/United States
- U01 AI068613/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1-AI068617/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
