Formation of a "Pre-mouth Array" from the Extreme Anterior Domain Is Directed by Neural Crest and Wnt/PCP Signaling
- PMID: 27425611
- PMCID: PMC4972695
- DOI: 10.1016/j.celrep.2016.06.073
Formation of a "Pre-mouth Array" from the Extreme Anterior Domain Is Directed by Neural Crest and Wnt/PCP Signaling
Abstract
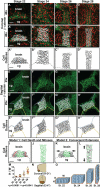
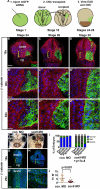
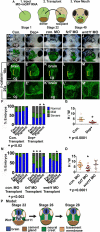
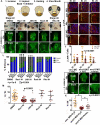
The mouth arises from the extreme anterior domain (EAD), a region where the ectoderm and endoderm are directly juxtaposed. Here, we identify a "pre-mouth array" in Xenopus that forms soon after the cranial neural crest has migrated to lie on either side of the EAD. Initially, EAD ectoderm comprises a wide and short epithelial mass that becomes narrow and tall with cells and nuclei changing shape, a characteristic of convergent extension. The resulting two rows of cells-the pre-mouth array-later split down the midline to surround the mouth opening. Neural crest is essential for convergent extension and likely signals to the EAD through the Wnt/planar cell polarity (PCP) pathway. Fzl7 receptor is locally required in EAD ectoderm, while Wnt11 ligand is required more globally. Indeed, heterologous cells expressing Wnt11 can elicit EAD convergent extension. The study reveals a precise cellular mechanism that positions and contributes to the future mouth.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Mouth development.Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):e275. doi: 10.1002/wdev.275. Epub 2017 May 17. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28514120 Free PMC article. Review.
-
Specific induction of cranial placode cells from Xenopus ectoderm by modulating the levels of BMP, Wnt, and FGF signaling.Genesis. 2015 Oct;53(10):652-9. doi: 10.1002/dvg.22881. Epub 2015 Aug 24. Genesis. 2015. PMID: 26249012
-
A novel role of the organizer gene Goosecoid as an inhibitor of Wnt/PCP-mediated convergent extension in Xenopus and mouse.Sci Rep. 2017 Feb 21;7:43010. doi: 10.1038/srep43010. Sci Rep. 2017. PMID: 28220837 Free PMC article.
-
Wnt proteins can direct planar cell polarity in vertebrate ectoderm.Elife. 2016 Sep 23;5:e16463. doi: 10.7554/eLife.16463. Elife. 2016. PMID: 27658614 Free PMC article.
-
Neural crest inducing signals.Adv Exp Med Biol. 2006;589:24-31. doi: 10.1007/978-0-387-46954-6_2. Adv Exp Med Biol. 2006. PMID: 17076273 Review.
Cited by
-
Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development.J Dev Biol. 2023 May 31;11(2):24. doi: 10.3390/jdb11020024. J Dev Biol. 2023. PMID: 37367478 Free PMC article.
-
Models of convergent extension during morphogenesis.Wiley Interdiscip Rev Dev Biol. 2018 Jan;7(1):e293. doi: 10.1002/wdev.293. Epub 2017 Sep 14. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 28906063 Free PMC article. Review.
-
Fgf signalling is required for gill slit formation in the skate, Leucoraja erinacea.Dev Biol. 2024 Feb;506:85-94. doi: 10.1016/j.ydbio.2023.11.008. Epub 2023 Nov 29. Dev Biol. 2024. PMID: 38040078 Free PMC article.
-
Formation of the initial kidney and mouth opening in larval amphioxus studied with serial blockface scanning electron microscopy (SBSEM).Evodevo. 2018 Jun 21;9:16. doi: 10.1186/s13227-018-0104-3. eCollection 2018. Evodevo. 2018. PMID: 29977493 Free PMC article.
-
Mouth development.Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):e275. doi: 10.1002/wdev.275. Epub 2017 May 17. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28514120 Free PMC article. Review.
References
-
- Carmona-Fontaine C. PhD Thesis. University College of London; 2011. Epub. http://carloscarmonafontaine.wikispaces.com/Thesis.
-
- Chapman S, Sawitzke A, Campbell D, Schoenwolf G. A three-dimensional atlas of pituitary gland development in the zebrafish. J Comp. Neurol. 2005;487:428–440. - PubMed
-
- Couly G, Le Douarin N. Mapping of the early neural primordium in Quail-Chick chimeras: 1. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev. Bio. 1985;110:422–439. - PubMed
-
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non- canonical Wnt signaling in neural crest migration. Dev. 2005;132:2587–2597. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

