Building the Evidence Base of Blood-Based Biomarkers for Early Detection of Cancer: A Rapid Systematic Mapping Review
- PMID: 27426280
- PMCID: PMC5006664
- DOI: 10.1016/j.ebiom.2016.07.004
Building the Evidence Base of Blood-Based Biomarkers for Early Detection of Cancer: A Rapid Systematic Mapping Review
Abstract
Background: The Early Cancer Detection Consortium is developing a blood-test to screen the general population for early identification of cancer, and has therefore conducted a systematic mapping review to identify blood-based biomarkers that could be used for early identification of cancer.
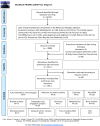
Methods: A mapping review with a systematic approach was performed to identify biomarkers and establish their state of development. Comprehensive searches of electronic databases Medline, Embase, CINAHL, the Cochrane library and Biosis were conducted in May 2014 to obtain relevant literature on blood-based biomarkers for cancer detection in humans. Screening of retrieved titles and abstracts was performed using an iterative sifting process known as "data mining". All blood based biomarkers, their relevant properties and characteristics, and their corresponding references were entered into an inclusive database for further scrutiny by the Consortium, and subsequent selection of biomarkers for rapid review. This systematic review is registered with PROSPERO (no. CRD42014010827).
Findings: The searches retrieved 19,724 records after duplicate removal. The data mining approach retrieved 3990 records (i.e. 20% of the original 19,724), which were considered for inclusion. A list of 814 potential blood-based biomarkers was generated from included studies. Clinical experts scrutinised the list to identify miss-classified and duplicate markers, also volunteering the names of biomarkers that may have been missed: no new markers were identified as a result. This resulted in a final list of 788 biomarkers.
Interpretation: This study is the first to systematically and comprehensively map blood biomarkers for early detection of cancer. Use of this rapid systematic mapping approach found a broad range of relevant biomarkers allowing an evidence-based approach to identification of promising biomarkers for development of a blood-based cancer screening test in the general population.
Keywords: Assay; Biomarker; Blood; Cancer; Diagnosis; Early detection; Systematic review.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Similar articles
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: a systematic mapping review.BMC Cancer. 2017 Oct 23;17(1):697. doi: 10.1186/s12885-017-3693-7. BMC Cancer. 2017. PMID: 29061138 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
Cited by
-
Identification of a three-miRNA panel in serum for bladder cancer diagnosis by a diagnostic test.Transl Cancer Res. 2022 May;11(5):1005-1016. doi: 10.21037/tcr-21-2611. Transl Cancer Res. 2022. PMID: 35706801 Free PMC article.
-
Networks Models of Actin Dynamics during Spermatozoa Postejaculatory Life: A Comparison among Human-Made and Text Mining-Based Models.Biomed Res Int. 2016;2016:9795409. doi: 10.1155/2016/9795409. Epub 2016 Aug 24. Biomed Res Int. 2016. PMID: 27642606 Free PMC article.
-
Biomarkers for Early Cancer Detection: A Landscape View of Recent Advancements, Spotlighting Pancreatic and Liver Cancers.ACS Pharmacol Transl Sci. 2024 Feb 14;7(3):586-613. doi: 10.1021/acsptsci.3c00346. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481702 Free PMC article. Review.
-
Human beings as islands of stability: Monitoring body states using breath profiles.Sci Rep. 2019 Nov 7;9(1):16167. doi: 10.1038/s41598-019-51417-0. Sci Rep. 2019. PMID: 31700057 Free PMC article.
-
Serum TNFRII: A promising biomarker for predicting the risk of subcentimetre lung adenocarcinoma.J Cell Mol Med. 2020 Apr;24(7):4150-4156. doi: 10.1111/jcmm.15071. Epub 2020 Feb 19. J Cell Mol Med. 2020. PMID: 32073741 Free PMC article.
References
-
- Barak V., Holdenrieder S., Nisman B., Stieber P. Relevance of circulating biomarkers for the therapy monitoring and follow-up investigations in patients with non-small cell lung cancer. Cancer Biomark. 2010;6(3–4):191–196. - PubMed
-
- Beije N., Jager A., Sleijfer S. Circulating tumor cell enumeration by the CellSearch system: the clinician's guide to breast cancer treatment? Cancer Treat. Rev. 2015;41(2):144–150. - PubMed
-
- Berruti A., Torta M., Piovesan A. Biochemical picture of bone metabolism in breast cancer patients with bone metastases. Anticancer Res. 1995;15(6B):2871–2875. - PubMed
-
- Clancy C., Joyce M.R., Kerin M.J. The use of circulating microRNAs as diagnostic biomarkers in colorectal cancer. Cancer Biomark. 2014 - PubMed
-
- Cochrane A.L., Holland W.W. Validation of screening procedures. Br. Med. Bull. 1971;27(1):3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources