In vivo matching of postsynaptic excitability with spontaneous synaptic inputs during formation of the rat calyx of Held synapse
- PMID: 27426483
- PMCID: PMC5199743
- DOI: 10.1113/JP272780
In vivo matching of postsynaptic excitability with spontaneous synaptic inputs during formation of the rat calyx of Held synapse
Abstract
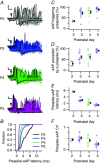
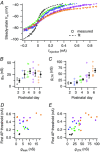
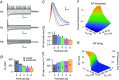
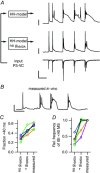
Key points: Neurons in the medial nucleus of the trapezoid body of anaesthetized rats of postnatal day (P)2-6 showed burst firing with a preferred interval of about 100 ms, which was stable, and a second preferred interval of 5-30 ms, which shortened during development. In 3 out of 132 cases, evidence for the presence of two large inputs was found. In vivo whole-cell recordings revealed that the excitability of the principal neuron and the size of its largest synaptic inputs were developmentally matched. At P2-4, action potentials were triggered by barrages of small synaptic events that summated to plateau potentials, while at later stages firing depended on a single, large and often prespike-associated input, which is probably the nascent calyx of Held. Simulations with a Hodgkin-Huxley-like model, which was based on fits of the intrinsic postsynaptic properties, suggested an essential role for the low-threshold potassium conductance in this transition.
Abstract: In the adult, principal neurons of the medial nucleus of the trapezoid body (MNTB) are typically contacted by a single, giant terminal called the calyx of Held, whereas during early development a principal neuron receives inputs from many axons. How these changes in innervation impact the postsynaptic activity has not yet been studied in vivo. We therefore recorded spontaneous inputs and intrinsic properties of principal neurons in anaesthetized rat pups during the developmental period in which the calyx forms. A characteristic bursting pattern could already be observed at postnatal day (P)2, before formation of the calyx. At this age, action potentials (APs) were triggered by barrages of summating EPSPs causing plateau depolarizations. In contrast, at P5, a single EPSP reliably triggered APs, resulting in a close match between pre- and postsynaptic firing. Postsynaptic excitability and the size of the largest synaptic events were developmentally matched. The developmental changes in intrinsic properties were estimated by fitting in vivo current injections to a Hodgkin-Huxley-type model of the principal neuron. Our simulations indicated that the developmental increases in Ih , low-threshold K+ channels and leak currents contributed to the reduction in postsynaptic excitability, but that low-threshold K+ channels specifically functioned as a dampening influence in the near-threshold range, thus precluding small inputs from triggering APs. Together, these coincident changes help to propagate bursting activity along the auditory brainstem, and are essential steps towards establishing the relay function of the calyx of Held synapse.
Keywords: excitability; response homeostasis; synapse formation.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures






Similar articles
-
Structure-function relation of the developing calyx of Held synapse in vivo.J Physiol. 2020 Oct;598(20):4603-4619. doi: 10.1113/JP279976. Epub 2020 Aug 6. J Physiol. 2020. PMID: 33439501 Free PMC article.
-
Developmental changes in intrinsic excitability of principal neurons in the rat medial nucleus of the trapezoid body.Dev Neurobiol. 2011 Apr;71(4):284-95. doi: 10.1002/dneu.20856. Dev Neurobiol. 2011. PMID: 21394932
-
SK Channels Regulate Resting Properties and Signaling Reliability of a Developing Fast-Spiking Neuron.J Neurosci. 2017 Nov 1;37(44):10738-10747. doi: 10.1523/JNEUROSCI.1243-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982705 Free PMC article.
-
The calyx of Held synapse: from model synapse to auditory relay.Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
-
Potassium currents and excitability in second-order auditory and vestibular neurons.J Neurosci Res. 1998 Sep 1;53(5):511-20. doi: 10.1002/(SICI)1097-4547(19980901)53:5<511::AID-JNR1>3.0.CO;2-C. J Neurosci Res. 1998. PMID: 9726422 Review.
Cited by
-
Using ephaptic coupling to estimate the synaptic cleft resistivity of the calyx of Held synapse.PLoS Comput Biol. 2021 Oct 26;17(10):e1009527. doi: 10.1371/journal.pcbi.1009527. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34699519 Free PMC article.
-
Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice.eNeuro. 2017 Dec 27;4(6):ENEURO.0264-17.2017. doi: 10.1523/ENEURO.0264-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29291238 Free PMC article.
-
Structure-function relation of the developing calyx of Held synapse in vivo.J Physiol. 2020 Oct;598(20):4603-4619. doi: 10.1113/JP279976. Epub 2020 Aug 6. J Physiol. 2020. PMID: 33439501 Free PMC article.
-
Priming central sound processing circuits through induction of spontaneous activity in the cochlea before hearing onset.Trends Neurosci. 2024 Jul;47(7):522-537. doi: 10.1016/j.tins.2024.04.007. Epub 2024 May 22. Trends Neurosci. 2024. PMID: 38782701 Free PMC article. Review.
-
Loss of C1q alters the auditory brainstem response.Front Cell Neurosci. 2024 Oct 2;18:1464670. doi: 10.3389/fncel.2024.1464670. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39416682 Free PMC article.
References
-
- Ankri N, Legendre P, Faber DS & Korn H (1994). Automatic detection of spontaneous synaptic responses in central neurons. J Neurosci Methods 52, 87–100. - PubMed
-
- Belleau ML & Warren RA (2000). Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol 84, 2204–2216. - PubMed
-
- Bergsman JB, De Camilli P & McCormick DA (2004). Multiple large inputs to principal cells in the mouse medial nucleus of the trapezoid body. J Neurophysiol 92, 545–552. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

