Cancer-associated isocitrate dehydrogenase mutations induce mitochondrial DNA instability
- PMID: 27427385
- PMCID: PMC5179947
- DOI: 10.1093/hmg/ddw195
Cancer-associated isocitrate dehydrogenase mutations induce mitochondrial DNA instability
Abstract
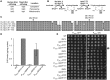
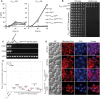
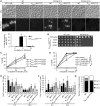
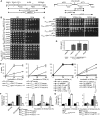
A major advance in understanding the progression and prognostic outcome of certain cancers, such as low-grade gliomas, acute myeloid leukaemia, and chondrosarcomas, has been the identification of early-occurring mutations in the NADP+-dependent isocitrate dehydrogenase genes IDH1 and IDH2 These mutations result in the production of the onco-metabolite D-2-hydroxyglutarate (2HG), thought to contribute to disease progression. To better understand the mechanisms of 2HG pathophysiology, we introduced the analogous glioma-associated mutations into the NADP+ isocitrate dehydrogenase genes (IDP1, IDP2, IDP3) in Saccharomyces cerevisiae Intriguingly, expression of the mitochondrial IDP1R148H mutant allele results in high levels of 2HG production as well as extensive mtDNA loss and respiration defects. We find no evidence for a reactive oxygen-mediated mechanism mediating this mtDNA loss. Instead, we show that 2HG production perturbs the iron sensing mechanisms as indicated by upregulation of the Aft1-controlled iron regulon and a concomitant increase in iron levels. Accordingly, iron chelation, or overexpression of a truncated AFT1 allele that dampens transcription of the iron regulon, suppresses the loss of respirative capacity. Additional suppressing factors include overexpression of the mitochondrial aldehyde dehydrogenase gene ALD5 or disruption of the retrograde response transcription factor RTG1 Furthermore, elevated α-ketoglutarate levels also suppress 2HG-mediated respiration loss; consistent with a mechanism by which 2HG contributes to mtDNA loss by acting as a toxic α-ketoglutarate analog. Our findings provide insight into the mechanisms that may contribute to 2HG oncogenicity in glioma and acute myeloid leukaemia progression, with the promise for innovative diagnostic and prognostic strategies and novel therapeutic modalities.
© The Author 2016. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations.PLoS One. 2011 Feb 4;6(2):e16812. doi: 10.1371/journal.pone.0016812. PLoS One. 2011. PMID: 21326614 Free PMC article.
-
Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas.Cancer Res. 2013 Jan 15;73(2):496-501. doi: 10.1158/0008-5472.CAN-12-2852. Epub 2012 Nov 30. Cancer Res. 2013. PMID: 23204232 Free PMC article.
-
Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.Nature. 2009 Dec 10;462(7274):739-44. doi: 10.1038/nature08617. Nature. 2009. PMID: 19935646 Free PMC article.
-
2-Hydroxyglutarate in Cancer Cells.Antioxid Redox Signal. 2020 Nov 1;33(13):903-926. doi: 10.1089/ars.2019.7902. Epub 2020 Jan 22. Antioxid Redox Signal. 2020. PMID: 31847543 Free PMC article. Review.
-
IDH mutations in glioma and acute myeloid leukemia.Trends Mol Med. 2010 Sep;16(9):387-97. doi: 10.1016/j.molmed.2010.07.002. Epub 2010 Aug 5. Trends Mol Med. 2010. PMID: 20692206 Review.
Cited by
-
Mitochondrial metabolism as a target for acute myeloid leukemia treatment.Cancer Metab. 2021 Apr 21;9(1):17. doi: 10.1186/s40170-021-00253-w. Cancer Metab. 2021. PMID: 33883040 Free PMC article. Review.
-
Targeting 2-oxoglutarate dehydrogenase for cancer treatment.Am J Cancer Res. 2022 Apr 15;12(4):1436-1455. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530286 Free PMC article. Review.
-
Identification of Iron Metabolism-Related Genes as Prognostic Indicators for Lower-Grade Glioma.Front Oncol. 2021 Sep 9;11:729103. doi: 10.3389/fonc.2021.729103. eCollection 2021. Front Oncol. 2021. PMID: 34568059 Free PMC article.
-
IDH Mutation Analysis in Glioma Patients by CADMA Compared with SNaPshot Assay and two Immunohistochemical Methods.Pathol Oncol Res. 2019 Jul;25(3):971-978. doi: 10.1007/s12253-018-0413-9. Epub 2018 Mar 19. Pathol Oncol Res. 2019. PMID: 29556922 Free PMC article.
-
IDH1 fine-tunes cap-dependent translation initiation.J Mol Cell Biol. 2019 Oct 25;11(10):816-828. doi: 10.1093/jmcb/mjz082. J Mol Cell Biol. 2019. PMID: 31408165 Free PMC article.
References
-
- Bleeker F.E., Lamba S., Leenstra S., Troost D., Hulsebos T., Vandertop W.P., Frattini M., Molinari F., Knowles M., Cerrato A., et al. (2009) IDH1 mutations at residue p.R132 (IDH1R132) occur frequently in high-grade gliomas but not in other solid tumors. Hum. Mutat., 30, 7–11. - PubMed
-
- Hartmann C., Meyer J., Balss J., Capper D., Mueller W., Christians A., Felsberg J., Wolter M., Mawrin C., Wick W., et al. (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol., 118, 469–474. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

