mTORC2 activity in brain cancer: Extracellular nutrients are required to maintain oncogenic signaling
- PMID: 27427440
- PMCID: PMC5501721
- DOI: 10.1002/bies.201600026
mTORC2 activity in brain cancer: Extracellular nutrients are required to maintain oncogenic signaling
Abstract
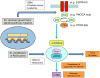
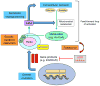
Mutations in growth factor receptor signaling pathways are common in cancer cells, including the highly lethal brain tumor glioblastoma (GBM) where they drive tumor growth through mechanisms including altering the uptake and utilization of nutrients. However, the impact of changes in micro-environmental nutrient levels on oncogenic signaling, tumor growth, and drug resistance is not well understood. We recently tested the hypothesis that external nutrients promote GBM growth and treatment resistance by maintaining the activity of mechanistic target of rapamycin complex 2 (mTORC2), a critical intermediate of growth factor receptor signaling, suggesting that altered cellular metabolism is not only a consequence of oncogenic signaling, but also potentially an important determinant of its activity. Here, we describe the studies that corroborate the hypothesis and propose others that derive from them. Notably, this line of reasoning raises the possibility that systemic metabolism may contribute to responsiveness to targeted cancer therapies.
Keywords: genetic-environment interaction; genetics; glioblastoma; mTOR; metabolic reprogramming.
© 2016 WILEY Periodicals, Inc.
Figures
Similar articles
-
mTORC2 and Metabolic Reprogramming in GBM: at the Interface of Genetics and Environment.Brain Pathol. 2015 Nov;25(6):755-9. doi: 10.1111/bpa.12307. Brain Pathol. 2015. PMID: 26526943 Free PMC article. Review.
-
Phosphorylation of the Hippo Pathway Component AMOTL2 by the mTORC2 Kinase Promotes YAP Signaling, Resulting in Enhanced Glioblastoma Growth and Invasiveness.J Biol Chem. 2015 Aug 7;290(32):19387-401. doi: 10.1074/jbc.M115.656587. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998128 Free PMC article.
-
mTORC2 in the center of cancer metabolic reprogramming.Trends Endocrinol Metab. 2014 Jul;25(7):364-73. doi: 10.1016/j.tem.2014.04.002. Epub 2014 May 21. Trends Endocrinol Metab. 2014. PMID: 24856037 Free PMC article.
-
ATP-site binding inhibitor effectively targets mTORC1 and mTORC2 complexes in glioblastoma.Int J Oncol. 2016 Mar;48(3):1045-52. doi: 10.3892/ijo.2015.3311. Epub 2015 Dec 28. Int J Oncol. 2016. PMID: 26719046
-
Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma.Adv Biol Regul. 2022 Jan;83:100854. doi: 10.1016/j.jbior.2021.100854. Epub 2021 Dec 6. Adv Biol Regul. 2022. PMID: 34996736 Review.
Cited by
-
mTOR Complexes as a Nutrient Sensor for Driving Cancer Progression.Int J Mol Sci. 2018 Oct 21;19(10):3267. doi: 10.3390/ijms19103267. Int J Mol Sci. 2018. PMID: 30347859 Free PMC article. Review.
-
Repurposing Potential of Riluzole as an ITAF Inhibitor in mTOR Therapy Resistant Glioblastoma.Int J Mol Sci. 2020 Jan 5;21(1):344. doi: 10.3390/ijms21010344. Int J Mol Sci. 2020. PMID: 31948038 Free PMC article.
-
Nuclear Functions of TOR: Impact on Transcription and the Epigenome.Genes (Basel). 2020 Jun 10;11(6):641. doi: 10.3390/genes11060641. Genes (Basel). 2020. PMID: 32532005 Free PMC article. Review.
-
New Insights into the Regulation of mTOR Signaling via Ca2+-Binding Proteins.Int J Mol Sci. 2023 Feb 15;24(4):3923. doi: 10.3390/ijms24043923. Int J Mol Sci. 2023. PMID: 36835331 Free PMC article. Review.
-
Crosstalk of Epigenetic and Metabolic Signaling Underpinning Glioblastoma Pathogenesis.Cancers (Basel). 2022 May 27;14(11):2655. doi: 10.3390/cancers14112655. Cancers (Basel). 2022. PMID: 35681635 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

