Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity
- PMID: 27427516
- PMCID: PMC6764831
- DOI: 10.1016/j.freeradbiomed.2016.07.009
Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity
Abstract
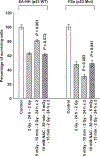
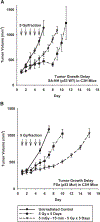
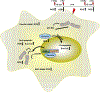
Exposure of cells to a dose of ionizing radiation as low as 5mGy can induce changes in radiation sensitivity expressed by cells exposed to subsequent higher doses at later times. This is referred to as an adaptive effect. We describe a unique survivin-associated adaptive response in which increased radiation resistance or sensitization of cells can be induced by exposure to 5mGy or to the reactive oxygen species (ROS) generating drug Emodin (1,3,8-trihydroxy-6-methylanthraquinone), a naturally occurring anthraquinone. The purpose of this study was to determine the role of ROS generating processes in affecting both the intracellular localization of the inhibitor of apoptosis protein survivin and its subsequent effect on radiation response in the presence or absence of the anti-oxidant N-acetyl-L-cysteine (NAC). Experiments were performed using two well characterized murine sarcomas: SA-NH p53 wild-type (WT) and FSa p53 mutant (Mut), grown either in culture or as solid tumors in the right hind legs of C3H mice. Doses of 5mGy or 50μM Emodin were used to induce changes in the response of these tumor cells to higher radiation exposures using a multi-dosing paradigm. Effects on radiation sensitivity were determined for SA-NH and FSa cells as a function of survivin translocation either to the cytoplasm or nucleus in the presence or absence of 10mM NAC treatment. In vitro survival assays (2Gy per fraction, two once daily fractions) and tumor growth delay (TGD) (5Gy per fraction, five once daily fractions) studies were performed. Intracellular localization of survivin was determined by enzyme-linked immunosorbent assay (ELISA) and correlated to survival response and treatment conditions. 2Gy alone had no effect on intracellular translocation of survivin. When preceded 15min earlier by 5mGy or Emodin exposures, survivin became elevated in the cytoplasm of p53 WT SA-NH as compared to the nuclei of p53 Mut FSa cells. SA-NH cells transfected with p53 small interfering RNA (siRNA), in contrast, responded similarly to p53 Mut FSa cells by becoming more radiation sensitive if exposed to 5mGy prior to each 2Gy irradiation. In contrast to their respective responses to five once daily 5Gy fractions, SA-NH tumors were protected by 5mGy exposures administered 15min prior to each daily 5Gy dose as evidenced by a more rapid growth (1.9 day decrease in TGD, P=0.032), while FSa tumors were sensitized, growing at a much slower rate (4.5 day increase in TGD, P<0.001). Exposure of SA-NH and FSa tumor cells to 10mM NAC inhibited the ability of 5mGy and Emodin to induce intracellular translocation of survivin and the corresponding altered adaptive survival response. The survivin-associated adaptive response can be induced following a multi-dosing scheme in which very low radiation doses are followed shortly thereafter by higher doses consistent with a standard image guided radiotherapy protocol that is currently widely used in the treatment of cancer. While induced by exposure to ROS generating stresses, the ultimate expression of changes in radiation response is dependent upon the bi-functionality of the tumor associated protein survivin and its intracellular translocation.
Keywords: Adaptive response; Emodin; Radiation response; Reactive oxygen species; Survivin; p53.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosure of potential conflicts of interest
Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
Figures




Similar articles
-
Altered expression of a metformin-mediated radiation response in SA-NH and FSa tumor cells treated under in vitro and in vivo growth conditions.Int J Radiat Biol. 2017 Jul;93(7):665-675. doi: 10.1080/09553002.2017.1304592. Epub 2017 Mar 28. Int J Radiat Biol. 2017. PMID: 28281393 Free PMC article.
-
TP53 Mutational Status and ROS Effect the Expression of the Survivin-Associated Radio-Adaptive Response.Radiat Res. 2017 Nov;188(5):579-590. doi: 10.1667/RR14831.1. Epub 2017 Aug 16. Radiat Res. 2017. PMID: 28813624 Free PMC article.
-
ROS modifiers and NOX4 affect the expression of the survivin-associated radio-adaptive response.Free Radic Biol Med. 2018 Aug 1;123:39-52. doi: 10.1016/j.freeradbiomed.2018.04.547. Epub 2018 Apr 13. Free Radic Biol Med. 2018. PMID: 29660403
-
Clinico-pathologic relevance of Survivin splice variant expression in cancer.Cancer Lett. 2013 Oct 10;339(2):167-74. doi: 10.1016/j.canlet.2013.06.007. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791888 Free PMC article. Review.
-
p53-mediated metabolic response to low doses of ionizing radiation.Int J Radiat Biol. 2023;99(6):934-940. doi: 10.1080/09553002.2022.2142983. Epub 2022 Dec 2. Int J Radiat Biol. 2023. PMID: 36357962 Review.
Cited by
-
Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives.Molecules. 2021 Oct 2;26(19):5997. doi: 10.3390/molecules26195997. Molecules. 2021. Retraction in: Molecules. 2023 Dec 14;28(24):8091. doi: 10.3390/molecules28248091. PMID: 34641542 Free PMC article. Retracted. Review.
-
Altered expression of a metformin-mediated radiation response in SA-NH and FSa tumor cells treated under in vitro and in vivo growth conditions.Int J Radiat Biol. 2017 Jul;93(7):665-675. doi: 10.1080/09553002.2017.1304592. Epub 2017 Mar 28. Int J Radiat Biol. 2017. PMID: 28281393 Free PMC article.
-
Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy.Int J Mol Sci. 2019 Oct 23;20(21):5267. doi: 10.3390/ijms20215267. Int J Mol Sci. 2019. PMID: 31652849 Free PMC article. Review.
-
TP53 Mutational Status and ROS Effect the Expression of the Survivin-Associated Radio-Adaptive Response.Radiat Res. 2017 Nov;188(5):579-590. doi: 10.1667/RR14831.1. Epub 2017 Aug 16. Radiat Res. 2017. PMID: 28813624 Free PMC article.
References
-
- Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A, Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double strand breaks in DNA, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med 53 (1) (1988) 39–47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

