The Integrated Role of Wnt/β-Catenin, N-Glycosylation, and E-Cadherin-Mediated Adhesion in Network Dynamics
- PMID: 27427963
- PMCID: PMC4948889
- DOI: 10.1371/journal.pcbi.1005007
The Integrated Role of Wnt/β-Catenin, N-Glycosylation, and E-Cadherin-Mediated Adhesion in Network Dynamics
Abstract
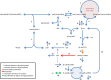
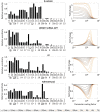
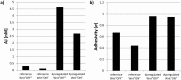
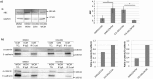
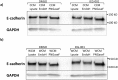
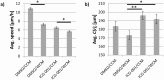
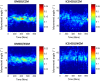
The cellular network composed of the evolutionarily conserved metabolic pathways of protein N-glycosylation, Wnt/β-catenin signaling pathway, and E-cadherin-mediated cell-cell adhesion plays pivotal roles in determining the balance between cell proliferation and intercellular adhesion during development and in maintaining homeostasis in differentiated tissues. These pathways share a highly conserved regulatory molecule, β-catenin, which functions as both a structural component of E-cadherin junctions and as a co-transcriptional activator of the Wnt/β-catenin signaling pathway, whose target is the N-glycosylation-regulating gene, DPAGT1. Whereas these pathways have been studied independently, little is known about the dynamics of their interaction. Here we present the first numerical model of this network in MDCK cells. Since the network comprises a large number of molecules with varying cell context and time-dependent levels of expression, it can give rise to a wide range of plausible cellular states that are difficult to track. Using known kinetic parameters for individual reactions in the component pathways, we have developed a theoretical framework and gained new insights into cellular regulation of the network. Specifically, we developed a mathematical model to quantify the fold-change in concentration of any molecule included in the mathematical representation of the network in response to a simulated activation of the Wnt/ β-catenin pathway with Wnt3a under different conditions. We quantified the importance of protein N-glycosylation and synthesis of the DPAGT1 encoded enzyme, GPT, in determining the abundance of cytoplasmic β-catenin. We confirmed the role of axin in β-catenin degradation. Finally, our data suggest that cell-cell adhesion is insensitive to E-cadherin recycling in the cell. We validate the model by inhibiting β-catenin-mediated activation of DPAGT1 expression and predicting changes in cytoplasmic β-catenin concentration and stability of E-cadherin junctions in response to DPAGT1 inhibition. We show the impact of pathway dysregulation through measurements of cell migration in scratch-wound assays. Collectively, our results highlight the importance of numerical analyses of cellular networks dynamics to gain insights into physiological processes and potential design of therapeutic strategies to prevent epithelial cell invasion in cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Näthke IS. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev Biol. 2004;20: 337–66. - PubMed
-
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24: 245–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

