Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape
- PMID: 27427982
- PMCID: PMC4966331
- DOI: 10.1172/JCI83416
Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape
Expression of concern in
-
Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape.J Clin Invest. 2019 Jul 2;129(8):3464. doi: 10.1172/JCI131246. eCollection 2019 Jul 2. J Clin Invest. 2019. PMID: 31264975 Free PMC article. No abstract available.
Abstract
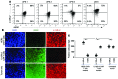
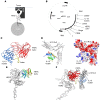
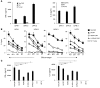
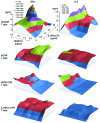
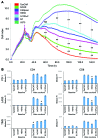
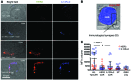
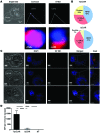
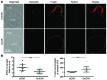
In preclinical models of glioblastoma, antigen escape variants can lead to tumor recurrence after treatment with CAR T cells that are redirected to single tumor antigens. Given the heterogeneous expression of antigens on glioblastomas, we hypothesized that a bispecific CAR molecule would mitigate antigen escape and improve the antitumor activity of T cells. Here, we created a CAR that joins a HER2-binding scFv and an IL13Rα2-binding IL-13 mutein to make a tandem CAR exodomain (TanCAR) and a CD28.ζ endodomain. We determined that patient TanCAR T cells showed distinct binding to HER2 or IL13Rα2 and had the capability to lyse autologous glioblastoma. TanCAR T cells exhibited activation dynamics that were comparable to those of single CAR T cells upon encounter of HER2 or IL13Rα2. We observed that TanCARs engaged HER2 and IL13Rα2 simultaneously by inducing HER2-IL13Rα2 heterodimers, which promoted superadditive T cell activation when both antigens were encountered concurrently. TanCAR T cell activity was more sustained but not more exhaustible than that of T cells that coexpressed a HER2 CAR and an IL13Rα2 CAR, T cells with a unispecific CAR, or a pooled product. In a murine glioblastoma model, TanCAR T cells mitigated antigen escape, displayed enhanced antitumor efficacy, and improved animal survival. Thus, TanCAR T cells show therapeutic potential to improve glioblastoma control by coengaging HER2 and IL13Rα2 in an augmented, bivalent immune synapse that enhances T cell functionality and reduces antigen escape.
Figures
Similar articles
-
Chimeric Antigen Receptor T Cells With Modified Interleukin-13 Preferentially Recognize IL13Rα2 and Suppress Malignant Glioma: A Preclinical Study.Front Immunol. 2021 Nov 8;12:715000. doi: 10.3389/fimmu.2021.715000. eCollection 2021. Front Immunol. 2021. PMID: 34819930 Free PMC article.
-
Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma.Mol Ther. 2013 Nov;21(11):2087-101. doi: 10.1038/mt.2013.185. Epub 2013 Aug 13. Mol Ther. 2013. PMID: 23939024 Free PMC article.
-
Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants.Cancer Immunol Res. 2017 Jul;5(7):571-581. doi: 10.1158/2326-6066.CIR-16-0376. Epub 2017 May 26. Cancer Immunol Res. 2017. PMID: 28550091 Free PMC article.
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
-
Present Status and Advances in Chimeric Antigen Receptor T Cell Therapy for Glioblastoma.Front Biosci (Landmark Ed). 2023 Sep 15;28(9):206. doi: 10.31083/j.fbl2809206. Front Biosci (Landmark Ed). 2023. PMID: 37796692 Review.
Cited by
-
Poliovirus receptor-based chimeric antigen receptor T cells combined with NK-92 cells exert potent activity against glioblastoma.J Natl Cancer Inst. 2024 Mar 7;116(3):389-400. doi: 10.1093/jnci/djad226. J Natl Cancer Inst. 2024. PMID: 37944044 Free PMC article.
-
Analysis and Augmentation of the Immunologic Bystander Effects of CAR T Cell Therapy in a Syngeneic Mouse Cancer Model.Mol Ther Oncolytics. 2020 Jul 15;18:360-371. doi: 10.1016/j.omto.2020.07.005. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32802940 Free PMC article.
-
Modern Advances in CARs Therapy and Creating a New Approach to Future Treatment.Int J Mol Sci. 2022 Nov 30;23(23):15006. doi: 10.3390/ijms232315006. Int J Mol Sci. 2022. PMID: 36499331 Free PMC article. Review.
-
Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma.Neurooncol Adv. 2022 Dec 22;5(1):vdac185. doi: 10.1093/noajnl/vdac185. eCollection 2023 Jan-Dec. Neurooncol Adv. 2022. PMID: 36751672 Free PMC article.
-
Folate Receptor-Alpha Targeted 7x19 CAR-γδT Suppressed Triple-Negative Breast Cancer Xenograft Model in Mice.J Oncol. 2022 Mar 7;2022:2112898. doi: 10.1155/2022/2112898. eCollection 2022. J Oncol. 2022. PMID: 35295709 Free PMC article.
References
-
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

