Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors
- PMID: 27427986
- PMCID: PMC4966323
- DOI: 10.1172/JCI85193
Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors
Abstract
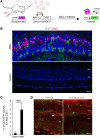
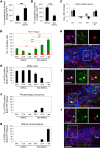
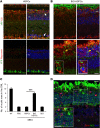
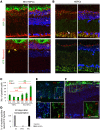
Vision impairments and blindness caused by retinitis pigmentosa result from severe neurodegeneration that leads to a loss of photoreceptors, the specialized light-sensitive neurons that enable vision. Although the mammalian nervous system is unable to replace neurons lost due to degeneration, therapeutic approaches to reprogram resident glial cells to replace retinal neurons have been proposed. Here, we demonstrate that retinal Müller glia can be reprogrammed in vivo into retinal precursors that then differentiate into photoreceptors. We transplanted hematopoietic stem and progenitor cells (HSPCs) into retinas affected by photoreceptor degeneration and observed spontaneous cell fusion events between Müller glia and the transplanted cells. Activation of Wnt signaling in the transplanted HSPCs enhanced survival and proliferation of Müller-HSPC hybrids as well as their reprogramming into intermediate photoreceptor precursors. This suggests that Wnt signaling drives the reprogrammed cells toward a photoreceptor progenitor fate. Finally, Müller-HSPC hybrids differentiated into photoreceptors. Transplantation of HSPCs with activated Wnt functionally rescued the retinal degeneration phenotype in rd10 mice, a model for inherited retinitis pigmentosa. Together, these results suggest that photoreceptors can be generated by reprogramming Müller glia and that this approach may have potential as a strategy for reversing retinal degeneration.
Figures


Similar articles
-
Lin28b stimulates the reprogramming of rat Müller glia to retinal progenitors.Exp Cell Res. 2017 Mar 1;352(1):164-174. doi: 10.1016/j.yexcr.2017.02.010. Epub 2017 Feb 9. Exp Cell Res. 2017. PMID: 28189638
-
Spontaneously Immortalised Nonhuman Primate Müller Glia Cell Lines as Source to Explore Retinal Reprogramming Mechanisms for Cell Therapies.J Cell Physiol. 2025 Jan;240(1):e31482. doi: 10.1002/jcp.31482. Epub 2024 Nov 28. J Cell Physiol. 2025. PMID: 39605294 Free PMC article.
-
Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells.Elife. 2025 Mar 6;13:RP102151. doi: 10.7554/eLife.102151. Elife. 2025. PMID: 40047533 Free PMC article.
-
Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish.Prog Retin Eye Res. 2014 May;40:94-123. doi: 10.1016/j.preteyeres.2013.12.007. Epub 2014 Jan 8. Prog Retin Eye Res. 2014. PMID: 24412518 Free PMC article. Review.
-
Prospects for the application of Müller glia and their derivatives in retinal regenerative therapies.Prog Retin Eye Res. 2021 Nov;85:100970. doi: 10.1016/j.preteyeres.2021.100970. Epub 2021 Apr 28. Prog Retin Eye Res. 2021. PMID: 33930561 Review.
Cited by
-
Zebrafish models of inherited retinal dystrophies.J Transl Genet Genom. 2022;6(1):95-110. doi: 10.20517/jtgg.2021.47. Epub 2022 Feb 8. J Transl Genet Genom. 2022. PMID: 35693295 Free PMC article.
-
Rare intercellular material transfer as a confound to interpreting inner retinal neuronal transplantation following internal limiting membrane disruption.Stem Cell Reports. 2023 Nov 14;18(11):2203-2221. doi: 10.1016/j.stemcr.2023.09.005. Epub 2023 Oct 5. Stem Cell Reports. 2023. PMID: 37802075 Free PMC article.
-
Subretinal versus intravitreal administration of human CD34+ bone marrow-derived stem cells in a rat model of inherited retinal degeneration.Ann Transl Med. 2021 Aug;9(15):1275. doi: 10.21037/atm-20-4662. Ann Transl Med. 2021. PMID: 34532412 Free PMC article.
-
Inherited Retinal Degenerations: Current Landscape and Knowledge Gaps.Transl Vis Sci Technol. 2018 Jul 18;7(4):6. doi: 10.1167/tvst.7.4.6. eCollection 2018 Jul. Transl Vis Sci Technol. 2018. PMID: 30034950 Free PMC article. No abstract available.
-
Harnessing the Neuroprotective Behaviors of Müller Glia for Retinal Repair.Front Biosci (Landmark Ed). 2022 May 30;27(6):169. doi: 10.31083/j.fbl2706169. Front Biosci (Landmark Ed). 2022. PMID: 35748245 Free PMC article. Review.
References
-
- Takahashi K, Ichisaka T, Yamanaka S. Identification of genes involved in tumor-like properties of embryonic stem cells. Methods Mol Biol. 2006;329:449–458. - PubMed
-
- Sanges D, Cosma MP. Reprogramming cell fate to pluripotency: the decision-making signalling pathways. Int J Dev Biol. 2010;54(11–12):1575–1587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

