Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants
- PMID: 27428110
- PMCID: PMC4948916
- DOI: 10.1371/journal.pone.0159033
Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants
Abstract
Background: Viral infection is a common cause of olfactory dysfunction. The complexities of studying post-viral olfactory loss in humans have impaired further progress in understanding the underlying mechanism. Recently, evidence from clinical studies has implicated Parainfluenza virus 3 as a causal agent. An animal model of post viral olfactory disorders (PVOD) would allow better understanding of disease pathogenesis and represent a major advance in the field.
Objective: To develop a mouse model of PVOD by evaluating the effects of Sendai virus (SeV), the murine counterpart of Parainfluenza virus, on olfactory function and regenerative ability of the olfactory epithelium.
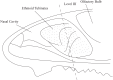
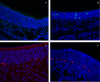
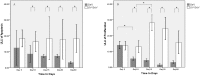
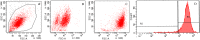
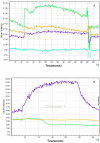
Methods: C57BL/6 mice (6-8 months old) were inoculated intranasally with SeV or ultraviolet (UV)-inactivated virus (UV-SeV). On days 3, 10, 15, 30 and 60 post-infection, olfactory epithelium was harvested and analyzed by histopathology and immunohistochemical detection of S-phase nuclei. We also measured apoptosis by TUNEL assay and viral load by real-time PCR. The buried food test (BFT) was used to measure olfactory function of mice at day 60. In parallel, cultured murine olfactory sensory neurons (OSNs) infected with SeV or UV-SeV were tested for odorant-mixture response by measuring changes in intracellular calcium concentrations indicated by fura-4 AM assay.
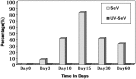
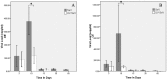
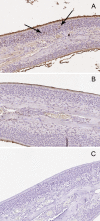
Results: Mice infected with SeV suffered from olfactory dysfunction, peaking on day 15, with no loss observed with UV-SeV. At 60 days, four out of 12 mice infected with SeV still had not recovered, with continued normal function in controls. Viral copies of SeV persisted in both the olfactory epithelium (OE) and the olfactory bulb (OB) for at least 60 days. At day 10 and after, both unit length labeling index (ULLI) of apoptosis and ULLI of proliferation in the SeV group was markedly less than the UV-SeV group. In primary cultured OSNs infected by SeV, the percentage of cells responding to mixed odors was markedly lower in the SeV group compared to UV-SeV (P = 0.007).
Conclusion: We demonstrate that SeV impairs olfaction, persists in OE and OB tissue, reduces their regenerative ability, and impairs the normal physiological function of OSNs without gross cytopathology. This mouse model shares key features of human post-viral olfactory loss, supporting its future use in studies of PVOD. Further testing and development of this model should allow us to clarify the pathophysiology of PVOD.
Conflict of interest statement
Figures



Similar articles
-
Persistent inflammation and hyperresponsiveness following viral rhinosinusitis.Laryngoscope. 2006 Jul;116(7):1236-40. doi: 10.1097/01.mlg.0000224526.43698.52. Laryngoscope. 2006. PMID: 16826067
-
Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients.Laryngoscope. 2007 Aug;117(8):1445-9. doi: 10.1097/MLG.0b013e318063e878. Laryngoscope. 2007. PMID: 17572640
-
Cigarette Smoke Delays Regeneration of the Olfactory Epithelium in Mice.Neurotox Res. 2016 Aug;30(2):213-24. doi: 10.1007/s12640-016-9617-5. Epub 2016 Mar 22. Neurotox Res. 2016. PMID: 27003941
-
[Advances in etiology and pathogenic mechanisms of postviral olfactory dysfunction].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019 May;33(5):477-480. doi: 10.13201/j.issn.1001-1781.2019.05.025. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019. PMID: 31163565 Review. Chinese.
-
Chronic sinusitis and olfactory dysfunction.Otolaryngol Clin North Am. 2004 Dec;37(6):1143-57, v-vi. doi: 10.1016/j.otc.2004.06.003. Otolaryngol Clin North Am. 2004. PMID: 15563907 Review.
Cited by
-
Is SARS-CoV-2 (COVID-19) postviral olfactory dysfunction (PVOD) different from other PVOD?World J Otorhinolaryngol Head Neck Surg. 2020 Nov;6(Suppl 1):S26-S32. doi: 10.1016/j.wjorl.2020.05.004. Epub 2020 May 19. World J Otorhinolaryngol Head Neck Surg. 2020. PMID: 32837756 Free PMC article. Review.
-
Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review.Open Forum Infect Dis. 2020 Jun 5;7(6):ofaa199. doi: 10.1093/ofid/ofaa199. eCollection 2020 Jun. Open Forum Infect Dis. 2020. PMID: 32548209 Free PMC article.
-
Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms.Trends Neurosci. 2023 Jan;46(1):75-90. doi: 10.1016/j.tins.2022.11.003. Epub 2022 Nov 16. Trends Neurosci. 2023. PMID: 36470705 Free PMC article. Review.
-
Innate immune signaling in the olfactory epithelium reduces odorant receptor levels: modeling transient smell loss in COVID-19 patients.medRxiv [Preprint]. 2020 Jun 16:2020.06.14.20131128. doi: 10.1101/2020.06.14.20131128. medRxiv. 2020. PMID: 32587994 Free PMC article. Preprint.
-
Effectiveness of platelet-rich plasma in long-lasting post-viral olfactory dysfunction: a case-series.Eur Arch Otorhinolaryngol. 2024 Nov;281(11):5841-5846. doi: 10.1007/s00405-024-08816-5. Epub 2024 Jul 11. Eur Arch Otorhinolaryngol. 2024. PMID: 38992193
References
-
- Seiden AM. Postviral olfactory loss. Otolaryngologic Clinics of North America 2004;37(6):1159–1166. - PubMed
-
- Leopold D. A Perplexing Olfactory Loss. Archives of Otolaryngology–Head & Neck Surgery 2000;126(6):803. - PubMed
-
- Sugiura M, Aiba T, Mori J, Nakai Y. An epidemiological study of postviral olfactory disorder. Acta Otolaryngol Suppl 1998;538:191–6. - PubMed
-
- Wang JH, Kwon HJ, Jang YJ. Detection of Parainfluenza Virus 3 in Turbinate Epithelial Cells of Postviral Olfactory Dysfunction Patients. The Laryngoscope 2007;117(8):1445–1449. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

