Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial
- PMID: 27428211
- PMCID: PMC4956805
- DOI: 10.1097/MD.0000000000004174
Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial
Abstract
Background: The ex vivo challenge assay is a bio-indicator of drug efficacy and was utilized in this randomized, placebo controlled trial as one of the exploratory endpoints. Fresh and cryopreserved tissues were evaluated for human immunodeficiency virus (HIV) infection and pharmacokinetic (PK)/pharmacodynamic (PD) relationships.
Methods: HIV-negative women used vaginal rings containing 25 mg dapivirine (DPV)/100 mg maraviroc (MVC) (n = 12), DPV only (n = 12), MVC only (n = 12), or placebo (n = 12) for 28 days. Blood plasma, cervicovaginal fluid (CVF), and cervical biopsies were collected for drug quantification and the ex vivo challenge assay; half (fresh) were exposed immediately to HIV while the other half were cryopreserved, thawed, then exposed to HIV. HIV replication was monitored by p24 enzyme-linked immunosorbent assay from culture supernatant. Data were log-transformed and analyzed by linear least squared regression, nonlinear Emax dose-response model and Satterthwaite t test.
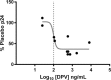
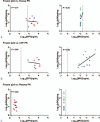
Results: HIV replication was greater in fresh compared to cryopreserved tissue (P = 0.04). DPV was detected in all compartments, while MVC was consistently detected only in CVF. Significant negative correlations between p24 and DPV levels were observed in fresh cervical tissue (P = 0.01) and CVF (P = 0.03), but not plasma. CVF MVC levels showed a significant negative correlation with p24 levels (P = 0.03); drug levels in plasma and tissue were not correlated with HIV suppression. p24 levels from cryopreserved tissue did not correlate to either drug from any compartment.
Conclusion: Fresh tissue replicated HIV to greater levels and defined PK/PD relationships while cryopreserved tissue did not. The ex vivo challenge assay using fresh tissue could prioritize drugs being considered for HIV prevention.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Abner SR, Guenthner PC, Guarner J, et al. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis 2005; 192:1545–1556. - PubMed
-
- Collins KB, Patterson BK, Naus GJ, et al. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 2000; 6:475–479. - PubMed
-
- Fletcher PS, Elliott J, Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006; 20:1237–1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

