Pig Liver Xenotransplantation: A Review of Progress Toward the Clinic
- PMID: 27428714
- PMCID: PMC5030131
- DOI: 10.1097/TP.0000000000001319
Pig Liver Xenotransplantation: A Review of Progress Toward the Clinic
Abstract
Experience with clinical liver xenotransplantation has largely involved the transplantation of livers from nonhuman primates. Experience with pig livers has been scarce. This brief review will be restricted to assessing the potential therapeutic impact of pig liver xenotransplantation in acute liver failure and the remaining barriers that currently do not justify clinical trials. A relatively new surgical technique of heterotopic pig liver xenotransplantation is described that might play a role in bridging a patient with acute liver failure until either the native liver recovers or a suitable liver allograft is obtained. Other topics discussed include the possible mechanisms for the development of the thrombocytopenis that rapidly occurs after pig liver xenotransplantation in a primate, the impact of pig complement on graft injury, the potential infectious risks, and potential physiologic incompatibilities between pig and human. There is cautious optimism that all of these problems can be overcome by judicious genetic manipulation of the pig. If liver graft survival could be achieved in the absence of thrombocytopenia or rejection for a period of even a few days, there may be a role for pig liver transplantation as a bridge to allotransplantation in carefully selected patients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

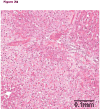


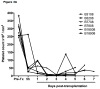

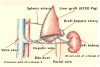


References
-
- UNOS. [Accessed October 20, 2015];United Network for Organ Sharing. Http://www.unos.org. Updated 2016.
-
- Hara H, Gridelli B, Lin YJ, Marcos A, Cooper DK. Liver xenografts for the treatment of acute liver failure: clinical and experimental experience and remaining immunologic barriers. Liver Transpl. 2008;14:425–434. - PubMed
-
- Levy MF, Crippin J, Sutton S, et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000;69:272–280. - PubMed
-
- Deschamps JY, Roux FA, Sai P, Gouin E. History of xenotransplantation. Xenotransplantation. 2005;12:91–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

