In Situ Investigation of a Self-Accelerated Cocrystal Formation by Grinding Pyrazinamide with Oxalic Acid
- PMID: 27428942
- PMCID: PMC6274108
- DOI: 10.3390/molecules21070917
In Situ Investigation of a Self-Accelerated Cocrystal Formation by Grinding Pyrazinamide with Oxalic Acid
Abstract
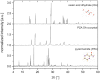
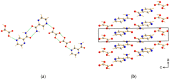
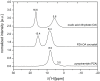
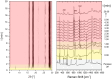
A new cocrystal of pyrazinamide with oxalic acid was prepared mechanochemically and characterized by PXRD, Raman spectroscopy, solid-state NMR spectroscopy, DTA-TG, and SEM. Based on powder X-ray diffraction data the structure was solved. The formation pathway of the reaction was studied in situ using combined synchrotron PXRD and Raman spectroscopy. Using oxalic acid dihydrate the initially neat grinding turned into a rapid self-accelerated liquid-assisted grinding process by the release of crystallization water. Under these conditions, the cocrystal was formed directly within two minutes.
Keywords: cocrystal; hydrate; in situ; mechanochemistry; pyrazinamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guidance for Industry, ANDA: Pharmaceutical Solid Polymorphism. Food and Drug Adminstration; Silver Spring, MD, USA: 2007.
-
- Aakeroy C.B., Salmon D.J. Building co-crystals with molecular sense and supramolecular sensibility. Crystengcomm. 2005;7:439–448. doi: 10.1039/b505883j. - DOI
-
- Aitipamula S., Banerjee R., Bansal A.K., Biradha K., Cheney M.L., Choudhury A.R., Desiraju G.R., Dikundwar A.G., Dubey R., Duggirala N., et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012;12:2147–2152. doi: 10.1021/cg3002948. - DOI
-
- Fischer F., Heidrich A., Greiser S., Benemann S., Rademann K., Emmerling F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016;16:1701–1707. doi: 10.1021/acs.cgd.5b01776. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

