Employing X-ray Photoelectron Spectroscopy for Determining Layer Homogeneity in Mixed Polar Self-Assembled Monolayers
- PMID: 27429041
- PMCID: PMC4976398
- DOI: 10.1021/acs.jpclett.6b01096
Employing X-ray Photoelectron Spectroscopy for Determining Layer Homogeneity in Mixed Polar Self-Assembled Monolayers
Abstract
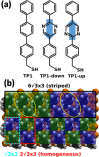
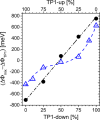
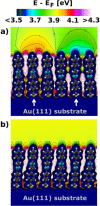
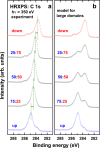
Self-assembled monolayers (SAMs) containing embedded dipolar groups offer the particular advantage of changing the electronic properties of a surface without affecting the SAM-ambient interface. Here we show that such systems can also be used for continuously tuning metal work functions by growing mixed monolayers consisting of molecules with different orientations of the embedded dipolar groups. To avoid injection hot-spots when using the SAM-modified electrodes in devices, a homogeneous mixing of the two components is crucial. We show that a combination of high-resolution X-ray photoelectron spectroscopy with state-of-the-art simulations is an ideal tool for probing the electrostatic homogeneity of the layers and thus for determining phase separation processes in polar adsorbate assemblies down to inhomogeneities at the molecular level.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Alloway D. M.; Hofmann M.; Smith D. L.; Gruhn N. E.; Graham A. L.; Colorado R.; Wysocki V. H.; Lee T. R.; Lee P. A.; Armstrong N. R. Interface Dipoles Arising from Self-Assembled Monolayers on Gold: UV-Photoemission Studies of Alkanethiols and Partially Fluorinated Alkanethiols. J. Phys. Chem. B 2003, 107, 11690–11699. 10.1021/jp034665+. - DOI
-
- de Boer B.; Hadipour A.; Mandoc M. M.; van Woudenbergh T.; Blom P. W. M. Tuning of Metal Work Functions with Self-Assembled Monolayers. Adv. Mater. 2005, 17, 621–625. 10.1002/adma.200401216. - DOI
-
- Marmont P.; Battaglini N.; Lang P.; Horowitz G.; Hwang J.; Kahn A.; Amato C.; Calas P. Improving Charge Injection in Organic Thin-Film Transistors with Thiol-Based Self-Assembled Monolayers. Org. Electron. 2008, 9, 419–424. 10.1016/j.orgel.2008.01.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

