Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments
- PMID: 27429252
- PMCID: PMC4963302
- DOI: 10.1016/j.biomaterials.2016.06.061
Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments
Abstract
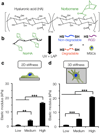
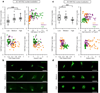
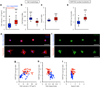
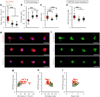
Improved fundamental understanding of how cells interpret microenvironmental signals is integral to designing better biomaterial therapies. YAP/TAZ are key mediators of mechanosensitive signaling; however, it is not clear how they are regulated by the complex interplay of microenvironmental factors (e.g., stiffness and degradability) and culture dimensionality. Using covalently crosslinked norbornene-functionalized hyaluronic acid (HA) hydrogels with controlled stiffness (via crosslink density) and degradability (via susceptibility of crosslinks to proteolysis), we found that human mesenchymal stem cells (MSCs) displayed increased spreading and YAP/TAZ nuclear localization when cultured atop stiffer hydrogels; however, the opposite trend was observed when MSCs were encapsulated within degradable hydrogels. When stiffness-matched hydrogels of reduced degradability were used, YAP/TAZ nuclear translocation was greater in cells that were able to spread, which was confirmed through pharmacological inhibition of YAP/TAZ and actin polymerization. Together, these data illustrate that YAP/TAZ signaling is responsive to hydrogel stiffness and degradability, but the outcome is dependent on the dimensionality of cell-biomaterial interactions.
Keywords: Degradation; Dimensionality; Hydrogel; Mechanotransduction; Stiffness; YAP/TAZ.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

