Modeling Melanoma In Vitro and In Vivo
- PMID: 27429258
- PMCID: PMC4934492
- DOI: 10.3390/healthcare2010027
Modeling Melanoma In Vitro and In Vivo
Abstract
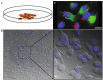
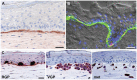
The behavior of melanoma cells has traditionally been studied in vitro in two-dimensional cell culture with cells adhering to plastic dishes. However, in order to mimic the three-dimensional architecture of a melanoma, as well as its interactions with the tumor microenvironment, there has been the need for more physiologically relevant models. This has been achieved by designing 3D in vitro models of melanoma, such as melanoma spheroids embedded in extracellular matrix or organotypic skin reconstructs. In vivo melanoma models have typically relied on the growth of tumor xenografts in immunocompromised mice. Several genetically engineered mouse models have now been developed which allow the generation of spontaneous melanoma. Melanoma models have also been established in other species such as zebrafish, which are more conducive to imaging and high throughput studies. We will discuss these models as well as novel techniques that are relevant to the study of the molecular mechanisms underlying melanoma progression.
Keywords: 3D models; animal models; genetically engineered mouse models (GEM); melanoma; spheroid models; xenograft models; zebrafish models.
Figures





References
-
- Balch C.M., Soong S.J., Gershenwald J.E., Thompson J.F., Reintgen D.S., Cascinelli N., Urist M., McMasters K.M., Ross M.I., Kirkwood J.M., et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 2001;19:3622–3634. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

