A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes
- PMID: 27430022
- PMCID: PMC4943462
- DOI: 10.1172/jci.insight.86177
A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes
Abstract
Vertebrate life critically depends on renal filtration and excretion of low molecular weight waste products. This process is controlled by a specialized cell-cell contact between podocyte foot processes: the slit diaphragm (SD). Using a comprehensive set of targeted KO mice of key SD molecules, we provided genetic, functional, and high-resolution ultrastructural data highlighting a concept of a flexible, dynamic, and multilayered architecture of the SD. Our data indicate that the mammalian SD is composed of NEPHRIN and NEPH1 molecules, while NEPH2 and NEPH3 do not participate in podocyte intercellular junction formation. Unexpectedly, homo- and heteromeric NEPHRIN/NEPH1 complexes are rarely observed. Instead, single NEPH1 molecules appear to form the lower part of the junction close to the glomerular basement membrane with a width of 23 nm, while single NEPHRIN molecules form an adjacent junction more apically with a width of 45 nm. In both cases, the molecules are quasiperiodically spaced 7 nm apart. These structural findings, in combination with the flexibility inherent to the repetitive Ig folds of NEPHRIN and NEPH1, indicate that the SD likely represents a highly dynamic cell-cell contact that forms an adjustable, nonclogging barrier within the renal filtration apparatus.
Figures



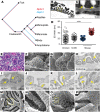
Comment in
-
Nephrotic syndrome: Insights into protein scaffolds of the slit diaphragm.Nat Rev Nephrol. 2016 Aug;12(8):441. doi: 10.1038/nrneph.2016.94. Epub 2016 Jun 27. Nat Rev Nephrol. 2016. PMID: 27345246 No abstract available.
References
-
- Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 2003;17(1):115–117. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

