Analysis of the population-level impact of co-administering ivermectin with albendazole or mebendazole for the control and elimination of Trichuris trichiura
- PMID: 27430028
- PMCID: PMC4946157
- DOI: 10.1016/j.parepi.2016.02.004
Analysis of the population-level impact of co-administering ivermectin with albendazole or mebendazole for the control and elimination of Trichuris trichiura
Abstract
Introduction: Soil-transmitted helminth (STH) infections are predominately controlled by providing children with preventive chemotherapy with either albendazole or mebendazole. However, neither has a high efficacy against Trichuris trichiura. This low efficacy limits the overall effectiveness of the current STH control programmes against T. trichiura. It has been demonstrated that co-administering ivermectin with albendazole or mebendazole significantly increases the efficacy of current treatments, which may increase the overall effectiveness of control programmes.
Methods: Using a STH transmission mathematical model, we evaluated the potential impact of co-administering ivermectin with albendazole or mebendazole to treat T. trichiura within a preventive chemotherapy programme targeting children (2-15 year olds). We evaluated the impact in terms of reduction in prevalent infections, mean worm burden, and prevalence of heavy infections.
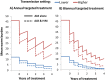
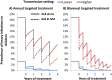
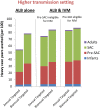
Results: Although the current treatment strategy reduced T. trichiura worm burden and prevalence of heavy infections, due to their poor efficacy the long term impact of preventive chemotherapy for children was smaller compared to the other STH. Co-administering ivermectin increased the projected impact of the preventive chemotherapy programme in terms of all three of the explored metrics, practically in high transmission settings. Furthermore, ivermectin co-administration greatly increased the feasibility of and timeframe for breaking transmission.
Conclusions: Co-administering ivermectin notably increased the projected impact of preventive chemotherapy in high transmission settings and increased the feasibility for breaking transmission. This has important implications for control programmes, some of which may be shifting focus from morbidity control to interruption of transmission, and some of which may be logistically unable to provide preventive chemotherapy twice a year as recommended. However, the benefit of co-administering ivermectin is limited by the fact that 2-5 year olds are often ineligible to receive treatment.
Keywords: ALB, albendazole; Control; ERRs, egg reduction rates; Elimination; IVM, ivermectin; Ivermectin co-administration; MBZ, mebendazole; Mass drug administration; Pre-SAC, preschool-aged; R0, basic reproductive number; SAC, school-aged children; STH, soil-transmitted helminth; Soil-transmitted helminth; Trichuris trichiura; WASH, water, sanitation and hygiene; WHO, World Health Organisation.
Figures



Similar articles
-
Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial.Lancet Infect Dis. 2015 Mar;15(3):277-84. doi: 10.1016/S1473-3099(14)71050-3. Epub 2015 Jan 12. Lancet Infect Dis. 2015. PMID: 25589326 Clinical Trial.
-
Efficacy and reinfection with soil-transmitted helminths 18-weeks post-treatment with albendazole-ivermectin, albendazole-mebendazole, albendazole-oxantel pamoate and mebendazole.Parasit Vectors. 2016 Mar 2;9:123. doi: 10.1186/s13071-016-1406-8. Parasit Vectors. 2016. PMID: 26935065 Free PMC article. Clinical Trial.
-
Efficacy and safety of albendazole alone versus albendazole in combination with ivermectin for the treatment of Trichuris trichiura infections: An open-label, randomized controlled superiority trial in south-western Uganda.PLoS Negl Trop Dis. 2024 Nov 26;18(11):e0012687. doi: 10.1371/journal.pntd.0012687. eCollection 2024 Nov. PLoS Negl Trop Dis. 2024. PMID: 39591454 Free PMC article. Clinical Trial.
-
Efficacy of Albendazole and Mebendazole Against Soil Transmitted Infections among Pre-School and School Age Children: A Systematic Review and Meta-Analysis.J Epidemiol Glob Health. 2024 Sep;14(3):884-904. doi: 10.1007/s44197-024-00231-7. Epub 2024 May 2. J Epidemiol Glob Health. 2024. PMID: 38696109 Free PMC article.
-
Efficacy of single-dose 500 mg mebendazole in soil-transmitted helminth infections: a review.J Helminthol. 2018 May;92(3):269-278. doi: 10.1017/S0022149X17000426. Epub 2017 Jul 18. J Helminthol. 2018. PMID: 28716158 Review.
Cited by
-
Investigating the Effectiveness of Current and Modified World Health Organization Guidelines for the Control of Soil-Transmitted Helminth Infections.Clin Infect Dis. 2018 Jun 1;66(suppl_4):S253-S259. doi: 10.1093/cid/ciy002. Clin Infect Dis. 2018. PMID: 29860285 Free PMC article.
-
Serious limitations of the current strategy to control Soil-Transmitted Helminths and added value of Ivermectin/Albendazole mass administration: A population-based observational study in Cameroon.PLoS Negl Trop Dis. 2020 Nov 3;14(11):e0008794. doi: 10.1371/journal.pntd.0008794. eCollection 2020 Nov. PLoS Negl Trop Dis. 2020. PMID: 33141853 Free PMC article.
-
A scoping review of transmission models for soil-transmitted helminth infections to underpin the development of a transmission model for Strongyloides stercoralis.Parasitology. 2024 Dec;151(14):1508-1521. doi: 10.1017/S0031182024001392. Parasitology. 2024. PMID: 39545321 Free PMC article.
-
Assessment of serum pharmacokinetics and urinary excretion of albendazole and its metabolites in human volunteers.PLoS Negl Trop Dis. 2018 Jan 18;12(1):e0005945. doi: 10.1371/journal.pntd.0005945. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29346367 Free PMC article.
-
Prospects for elimination of soil-transmitted helminths.Curr Opin Infect Dis. 2017 Oct;30(5):482-488. doi: 10.1097/QCO.0000000000000395. Curr Opin Infect Dis. 2017. PMID: 28700363 Free PMC article. Review.
References
-
- 25 Years: The MECTIZAN® Donation Program [http://www.merck.com/about/featured-stories/mectizan1.html].
-
- Anderson R.M., May R.M. Helminth infections of humans: mathematical models, population dynamics, and control. Adv. Parasitol. 1985;24:1–101. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

