Pilot Randomized trial of Fibrinogen in Trauma Haemorrhage (PRooF-iTH): study protocol for a randomized controlled trial
- PMID: 27430210
- PMCID: PMC4949907
- DOI: 10.1186/s13063-016-1439-5
Pilot Randomized trial of Fibrinogen in Trauma Haemorrhage (PRooF-iTH): study protocol for a randomized controlled trial
Abstract
Background: Haemorrhage remains a leading cause of morbidity and mortality in trauma patients. Fibrinogen is an essential endogenous component of haemostasis and the plasma level is associated with bleeding, transfusion and outcome. Fibrinogen concentrate is widely used to correct acquired hypofibrinogenaemia, recommended by several international guidelines for the treatment of trauma patients, but evidence is lacking regarding the treatment safety and efficacy. We aim to assess the efficacy and safety of an immediate pre-emptive first-line treatment with fibrinogen concentrate in patients with trauma haemorrhage in need of haemostatic resuscitation.
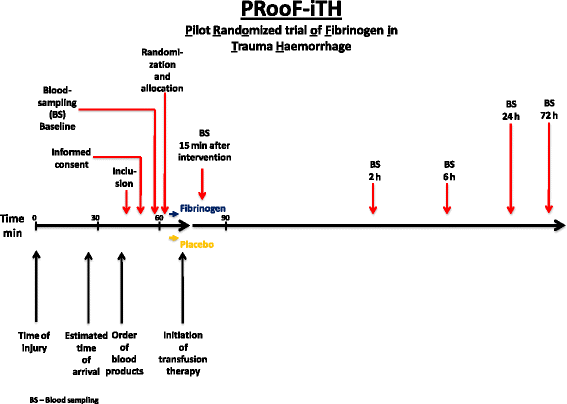
Methods/design: This is a single-centre, randomized (1:1, active:placebo), placebo-controlled, double-blinded, investigator-initiated phase II trial. The trial population consists of 40 adult patients (>18 years) with traumatic, critical bleeding admitted to the Level 1 Trauma Centre at Rigshospitalet in Copenhagen, with immediate need for blood transfusion on arrival and an expected need for haemostatic resuscitation with multiple transfusions during the initial resuscitation. Patients will receive either pre-emptive administration of a bolus dose of 60-70 mg/kg fibrinogen concentrate (Riastap®) or placebo 0.9 % saline in equal volume to active treatment, both given as intravenous infusion blinded for the person administering the infusion. The primary end point is the change in thrombelastograph (TEG®) functional fibrinogen maximum amplitude in millimetres at 15 min after the intervention. The follow-up period on safety events and mortality will be until day 30. To detect a difference in the change from baseline to the 15-minute post-randomization measurement of 6-8 mm in TEG® functional fibrinogen maximum amplitude with a power of 0.90 and alpha of 0.05, we require 19 patients in each group. We have chosen to include 40 patients, 20 evaluable patients in each randomization group in case of attrition, in the present trial.
Discussion: Patients considered to be included in the trial will temporarily have a compromised consciousness because of the acute, critical bleeding related to trauma, so scientific guardians will co-sign the informed consent form. Next of kin and the patients' general practitioner or the patients will co-sign as soon as possible. This trial will test whether immediate pre-emptive fibrinogen concentrate administered to adult trauma patients as first-line treatment of trauma haemorrhage will increase the clot strength as evaluated by thrombelastography, transfusion requirements and survival in patients receiving haemostatic resuscitation according to current standard of care.
Trial registration: EudraCT no. 2014-003978-16 (22/1 2015); ClinicalTrials.gov: NCT02344069 . Registered on 14 January 2015. Trial protocol version 4.2 (23-12-2014).
Keywords: Fibrinogen; Haemorrhage; Haemostatic Resuscitation; Thrombelastography; Trauma.
Similar articles
-
The FIB-PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial.Trials. 2012 Jul 17;13:110. doi: 10.1186/1745-6215-13-110. Trials. 2012. PMID: 22805300 Free PMC article. Clinical Trial.
-
Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial.Crit Care. 2018 Jun 18;22(1):164. doi: 10.1186/s13054-018-2086-x. Crit Care. 2018. PMID: 29914530 Free PMC article. Clinical Trial.
-
iTACTIC - implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy: study protocol for a multicentre, randomised controlled trial.Trials. 2017 Oct 18;18(1):486. doi: 10.1186/s13063-017-2224-9. Trials. 2017. PMID: 29047413 Free PMC article. Clinical Trial.
-
Use of fibrinogen concentrate for trauma-related bleeding: A systematic-review and meta-analysis.J Trauma Acute Care Surg. 2020 Dec;89(6):1212-1224. doi: 10.1097/TA.0000000000002920. J Trauma Acute Care Surg. 2020. PMID: 32890340
-
Fibrinogen concentrates for bleeding trauma patients: what is the evidence?Vox Sang. 2011 Oct;101(3):185-90. doi: 10.1111/j.1423-0410.2011.01478.x. Epub 2011 May 3. Vox Sang. 2011. PMID: 21535437 Review.
Cited by
-
The impact of blood product ratio and procoagulant therapy on the development of thromboembolic events in severely injured hemorrhaging trauma patients.Transfusion. 2020 Aug;60(8):1873-1882. doi: 10.1111/trf.15917. Epub 2020 Jun 24. Transfusion. 2020. PMID: 32579252 Free PMC article.
-
A retrospective register study comparing fibrinogen treated trauma patients with an injury severity score matched control group.Scand J Trauma Resusc Emerg Med. 2020 Jan 21;28(1):5. doi: 10.1186/s13049-019-0695-2. Scand J Trauma Resusc Emerg Med. 2020. PMID: 31964405 Free PMC article.
-
Pre-emptive administration of fibrinogen concentrate contributes to improved prognosis in patients with severe trauma.Trauma Surg Acute Care Open. 2016 Dec 2;1(1):e000037. doi: 10.1136/tsaco-2016-000037. eCollection 2016. Trauma Surg Acute Care Open. 2016. PMID: 29766069 Free PMC article.
-
The use of fibrinogen concentrate for the management of trauma-related bleeding: a systematic review and meta-analysis.Blood Transfus. 2017 Jul;15(4):318-324. doi: 10.2450/2017.0094-17. Blood Transfus. 2017. PMID: 28661856 Free PMC article.
-
Dynamics of fibrinogen in acute phases of trauma.J Intensive Care. 2017 Jan 20;5(1):3. doi: 10.1186/s40560-016-0199-3. J Intensive Care. 2017. PMID: 34798699 Free PMC article. Review.
References
-
- Johansson PI, Sorensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, et al. Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013;53:3088–99. doi: 10.1111/trf.12214. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical