Salivary pellets induce a pro-inflammatory response involving the TLR4-NF-kB pathway in gingival fibroblasts
- PMID: 27430277
- PMCID: PMC4948095
- DOI: 10.1186/s12903-016-0229-5
Salivary pellets induce a pro-inflammatory response involving the TLR4-NF-kB pathway in gingival fibroblasts
Abstract
Background: Whole saliva provokes a substantial pro-inflammatory response in gingival fibroblasts. This raises the question whether the salivary pellet, which is used for diagnostic purposes, also has a pro-inflammatory capacity and, if yes, what the underlying mechanisms at the molecular level are.
Methods: We examined the ability of extensively washed salivary pellets to provoke the expression of chemokines in gingival fibroblasts by real-time polymerase chain reaction and immunoassays. Protein composition was determined with proteomic analysis. Endotoxins were analyzed by a Limulus assay and removed by affinity chromatography. The inhibitors TAK-242 and BAY11-7082 were used to determine the involvement of the TLR4 and NF-kB signaling, respectively. Western blot was performed to detect phosphorylated p65.
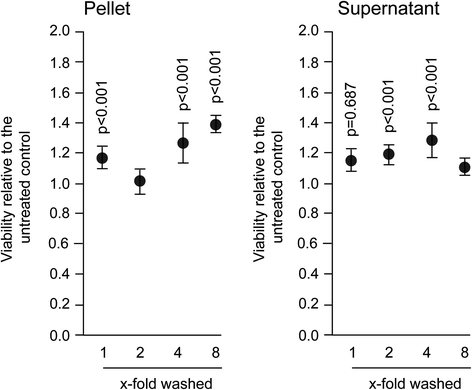
Results: The experiments show that salivary pellets and the corresponding washing solution contain pro-inflammatory activity without impairing cell viability. Proteomic analysis revealed proteins with a binding capacity for lipopolysaccharides, and the Limulus assay indicated the presence of endotoxin in the salivary pellets. Blocking TLR4 with TAK-242 and depletion of endotoxins both lowered the capacity of salivary pellets to increase chemokine expression and phosphorylation of p65. BAY11-7082 suppressed chemokine expression in response to the salivary pellets. Autoclaving salivary pellets also reduced their pro-inflammatory activity.
Conclusions: The data support the molecular mechanism of a TLR4-NF-kB-dependent pro-inflammatory response of the gingival fibroblasts exposed to preparations of washed salivary pellets. Together, the data indicate that the salivary pellet is rich in endotoxin but it is mainly a heat labile fraction that accounts for the chemokine expression in the bioassay.
Keywords: Gingival fibroblast; Inflammation; Lipopolysaccharide; Salivary pellet; Toll-like receptor.
Figures





Similar articles
-
Chemokine expression of oral fibroblasts and epithelial cells in response to artificial saliva.Clin Oral Investig. 2016 Jun;20(5):1035-42. doi: 10.1007/s00784-015-1582-5. Epub 2015 Sep 5. Clin Oral Investig. 2016. PMID: 26342602
-
Tormentic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts via inhibition of TLR4-mediated NF-κB and MAPK signalling pathway.Arch Oral Biol. 2015 Sep;60(9):1327-32. doi: 10.1016/j.archoralbio.2015.05.005. Epub 2015 May 22. Arch Oral Biol. 2015. PMID: 26123747
-
[Effect of resveratrol on expression of TLR4 and inflammatory factors in gingival epithelial cells under high glucose environment].Shanghai Kou Qiang Yi Xue. 2017 Feb;26(1):32-36. Shanghai Kou Qiang Yi Xue. 2017. PMID: 28474063 Chinese.
-
Differential expression of Toll-like receptor 4 in healthy and diseased human gingiva.J Periodontal Res. 2014 Dec;49(6):845-54. doi: 10.1111/jre.12173. Epub 2014 Mar 12. J Periodontal Res. 2014. PMID: 24620831
-
Interferon-γ stimulates CD14, TLR2 and TLR4 mRNA expression in gingival fibroblasts increasing responsiveness to bacterial challenge.Arch Oral Biol. 2016 Jan;61:36-43. doi: 10.1016/j.archoralbio.2015.10.005. Epub 2015 Oct 9. Arch Oral Biol. 2016. PMID: 26513680
Cited by
-
Effects of Saliva From Periodontally Healthy and Diseased Subjects on Barrier Function and the Inflammatory Response in in vitro Models of the Oral Epithelium.Front Oral Health. 2022 Jan 5;2:815728. doi: 10.3389/froh.2021.815728. eCollection 2021. Front Oral Health. 2022. PMID: 35048079 Free PMC article.
-
Astragaloside IV Alleviates the Myocardial Damage Induced by Lipopolysaccharide via the Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-κB)/Proliferator-Activated Receptor α (PPARα) Signaling Pathway.Med Sci Monit. 2019 Sep 23;25:7158-7168. doi: 10.12659/MSM.916030. Med Sci Monit. 2019. PMID: 31545785 Free PMC article.
-
Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update.Proteomes. 2016 Dec 15;4(4):41. doi: 10.3390/proteomes4040041. Proteomes. 2016. PMID: 28248250 Free PMC article. Review.
-
Extracellular CIRP Induces an Inflammatory Phenotype in Pulmonary Fibroblasts via TLR4.Front Immunol. 2021 Jul 23;12:721970. doi: 10.3389/fimmu.2021.721970. eCollection 2021. Front Immunol. 2021. PMID: 34367191 Free PMC article.
-
Expression of LncRNAs in anterior capsule of lens in patients with pathologic myopia complicated with cataract.Int Ophthalmol. 2024 Dec 16;45(1):10. doi: 10.1007/s10792-024-03366-5. Int Ophthalmol. 2024. PMID: 39680214
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

