Concurrent transcranial direct current stimulation and progressive resistance training in Parkinson's disease: study protocol for a randomised controlled trial
- PMID: 27430304
- PMCID: PMC4949761
- DOI: 10.1186/s13063-016-1461-7
Concurrent transcranial direct current stimulation and progressive resistance training in Parkinson's disease: study protocol for a randomised controlled trial
Abstract
Background: Parkinson's disease (PD) results from a loss of dopamine in the brain, leading to movement dysfunctions such as bradykinesia, postural instability, resting tremor and muscle rigidity. Furthermore, dopamine deficiency in PD has been shown to result in maladaptive plasticity of the primary motor cortex (M1). Progressive resistance training (PRT) is a popular intervention in PD that improves muscular strength and results in clinically significant improvements on the Unified Parkinson's Disease Rating Scale (UPDRS). In separate studies, the application of anodal transcranial direct current stimulation (a-tDCS) to the M1 has been shown to improve motor function in PD; however, the combined use of tDCS and PRT has not been investigated.
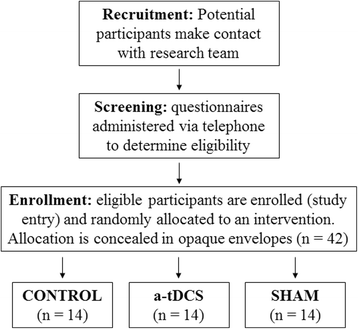
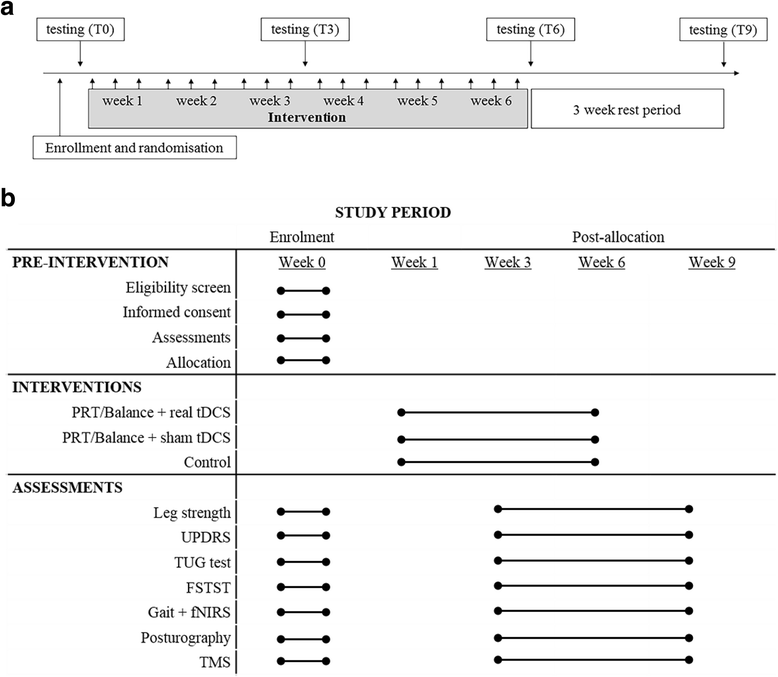
Methods/design: We propose a 6-week, double-blind randomised controlled trial combining M1 tDCS and PRT of the lower body in participants (n = 42) with moderate PD (Hoehn and Yahr scale score 2-4). Supervised lower body PRT combined with functional balance tasks will be performed three times per week with concurrent a-tDCS delivered at 2 mA for 20 minutes (a-tDCS group) or with sham tDCS (sham group). Control participants will receive standard care (control group). Outcome measures will include functional strength, gait speed and variability, balance, neurophysiological function at rest and during movement execution, and the UPDRS motor subscale, measured at baseline, 3 weeks (during), 6 weeks (post), and 9 weeks (retention). Ethical approval has been granted by the Deakin University Human Research Ethics Committee (project number 2015-014), and the trial has been registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615001241527).
Discussion: This will be the first randomised controlled trial to combine PRT and a-tDCS targeting balance and gait in people with PD. The study will elucidate the functional, clinical and neurophysiological outcomes of combined PRT and a-tDCS. It is hypothesised that combined PRT and a-tDCS will significantly improve lower limb strength, postural sway, gait speed and stride variability compared with PRT with sham tDCS. Further, we hypothesise that pre-frontal cortex activation during dual-task cognitive and gait/balance activities will be reduced, and that M1 excitability and inhibition will be augmented, following the combined PRT and a-tDCS intervention.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12615001241527 . Registered on 12 November 2015.
Keywords: Balance; Gait; Neuroplasticity; Non-invasive brain stimulation; Parkinson’s disease; fNIRS.
Figures
Similar articles
-
Concurrent exergaming and transcranial direct current stimulation to improve balance in people with Parkinson's disease: study protocol for a randomised controlled trial.Trials. 2018 Jul 16;19(1):387. doi: 10.1186/s13063-018-2773-6. Trials. 2018. PMID: 30012175 Free PMC article.
-
Effects of transcranial direct current stimulation on gait in people with Parkinson's disease: study protocol for a randomized, controlled clinical trial.Trials. 2018 Nov 29;19(1):661. doi: 10.1186/s13063-018-2982-z. Trials. 2018. PMID: 30486849 Free PMC article.
-
Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson's disease: A double blind, sham-controlled study.Gait Posture. 2021 Feb;84:11-16. doi: 10.1016/j.gaitpost.2020.11.012. Epub 2020 Nov 16. Gait Posture. 2021. PMID: 33260076
-
Effects of transcranial direct current stimulation alone and in combination with rehabilitation therapies on gait and balance among individuals with Parkinson's disease: a systematic review and meta-analysis.J Neuroeng Rehabil. 2024 Feb 19;21(1):27. doi: 10.1186/s12984-024-01311-2. J Neuroeng Rehabil. 2024. PMID: 38373966 Free PMC article.
-
Transcranial direct current stimulation provides no clinically important benefits over walking training for improving walking in Parkinson's disease: a systematic review.J Physiother. 2021 Jul;67(3):190-196. doi: 10.1016/j.jphys.2021.06.003. Epub 2021 Jun 17. J Physiother. 2021. PMID: 34147400
Cited by
-
Using non-invasive transcranial stimulation to improve motor and cognitive function in Parkinson's disease: a systematic review and meta-analysis.Sci Rep. 2017 Nov 1;7(1):14840. doi: 10.1038/s41598-017-13260-z. Sci Rep. 2017. PMID: 29093455 Free PMC article.
-
A Description and Critical Analysis of the Therapeutic Uses of Transcranial Direct Current Stimulation: Implications for Clinical Practice and Research.Nursing (Auckl). 2016;6:23-31. doi: 10.2147/NRR.S115627. Epub 2016 Sep 14. Nursing (Auckl). 2016. PMID: 27738595 Free PMC article.
References
-
- Brozova H, Stochl J, Roth J, et al. Fear of falling has greater influence than other aspects of gait disorders on quality of life in patients with Parkinson’s disease. Neuro Endocrinol Lett. 2009;30:453–7. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

