Neuroimaging of epilepsy
- PMID: 27430454
- PMCID: PMC5256664
- DOI: 10.1016/B978-0-444-53486-6.00051-X
Neuroimaging of epilepsy
Abstract
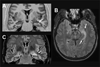
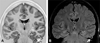
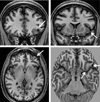
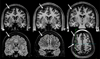
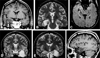
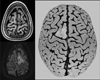
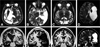
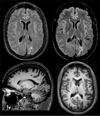
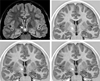
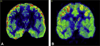
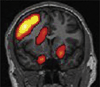
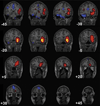
Imaging is pivotal in the evaluation and management of patients with seizure disorders. Elegant structural neuroimaging with magnetic resonance imaging (MRI) may assist in determining the etiology of focal epilepsy and demonstrating the anatomical changes associated with seizure activity. The high diagnostic yield of MRI to identify the common pathological findings in individuals with focal seizures including mesial temporal sclerosis, vascular anomalies, low-grade glial neoplasms and malformations of cortical development has been demonstrated. Positron emission tomography (PET) is the most commonly performed interictal functional neuroimaging technique that may reveal a focal hypometabolic region concordant with seizure onset. Single photon emission computed tomography (SPECT) studies may assist performance of ictal neuroimaging in patients with pharmacoresistant focal epilepsy being considered for neurosurgical treatment. This chapter highlights neuroimaging developments and innovations, and provides a comprehensive overview of the imaging strategies used to improve the care and management of people with epilepsy.
Keywords: 18F-fluorodeoxyglucose–positron emission tomography (18F-FDG-PET); Epilepsy; computed tomography (CT); drug-resistant focal epilepsy; ictal single photon emission computed tomography (SPECT); magnetic resonance imaging (MRI); surgical treatment of epilepsy.
© 2016 Elsevier B.V. All rights reserved.
Figures
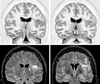

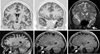
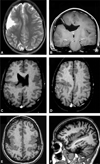

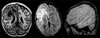
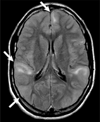
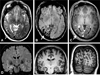
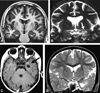
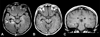



References
-
- Ahnlide J-A, Rosén I, Lindén-Mickelsson Tech P, et al. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia. 2007;48:579–588. - PubMed
-
- Andermann F. Rasmussen’s syndrome. Stoneham, MA: Butterworth-Heinemann; 1991. Chronic encephalitis and epilepsy.
-
- Andersen AR. 99mTc-D, L-hexamethylene-propyleneamine oxime (99mTc-HMPAO): basic kinetic studies of a tracer of cerebral blood flow. Cerebrovasc Brain Metab Rev. 1989;1:288–318. - PubMed
-
- Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446–450. - PubMed
-
- Banati RB, Goerres GW, Myers R, et al. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology. 1999;53:2199–2203. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

