Organizing the Donation of Convalescent Plasma for a Therapeutic Clinical Trial on Ebola Virus Disease: The Experience in Guinea
- PMID: 27430546
- PMCID: PMC5014273
- DOI: 10.4269/ajtmh.15-0890
Organizing the Donation of Convalescent Plasma for a Therapeutic Clinical Trial on Ebola Virus Disease: The Experience in Guinea
Abstract
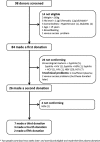
Although convalescent plasma (CP) transfusion was prioritized among potential Ebola treatments by the World Health Organization, there were concerns on the feasibility of its implementation. We report on the successful organization of donor mobilization and plasma collection as part of the Ebola-Tx clinical trial from November 2014 to July 2015 in Conakry, Guinea. Project implementation registers, tools and reports, mission reports, and minutes of research team meetings were used to reconstruct the sequence of events on how donor mobilization was organized, plasmapheresis was set up, and how effective this approach was in collecting CP. An initial needs assessment of the Guinean National Blood Transfusion Center resulted in targeted training of staff on site, resulting in autonomy and independent production of CP within 3 months. The Conakry Ebola Survivors Association played a direct role in donor mobilization and organization of CP donations. A total of 98 Ebola survivors were screened for plasma donation, of which 84 were found eligible for plasmapheresis. Of these, 26 (30.9%) were excluded. The remaining 58 donors made a total of 90 donations, corresponding to 50.9 L of CP. This sufficed to treat the 99 eligible patients enrolled in the trial. Within a poor resource emergency context, transfusion capacity could be rapidly improved through the strengthening of local capacities and gradual transfer of skills coupled with active involvement of Ebola survivors. However, large-scale plasma collection or multisite studies may require further adaptations of both strategy and logistics. The Ebola-Tx trial was funded by the European Union and others.
© The American Society of Tropical Medicine and Hygiene.
Figures

References
-
- World Health Organization . Statement on the WHO Consultation on Potential Ebola Therapies and Vaccines. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/mediacentre/news/statements/2014/ebola-therapies-cons... Available at. Accessed May 19, 2015.
-
- Gulland A. First Ebola treatment is approved by WHO. BMJ. 2014;349:g5539. - PubMed
-
- World Health Organization . Use of Convalescent Whole Blood or Plasma Collected from Patients Recovered from Ebola Virus Disease for Transfusion, as an Empirical Treatment During Outbreaks. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/csr/resources/publications/ebola/convalescent-treatme... Interim guidance for national health authorities and blood transfusion services. Available at. Accessed May 19, 2015.
-
- Bannister-Tyrrell M, Gryseels C, Delamou A, D'Alessandro U, van Griensven J, Grietens KP. Tx Trial Platform Blood as medicine: social meanings of blood and the success of Ebola trials. Lancet. 2015;385:420. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

