CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: A multi-center, multi-national, randomized controlled trial - Study protocol
- PMID: 27430714
- PMCID: PMC4950103
- DOI: 10.1186/s12884-016-0961-5
CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: A multi-center, multi-national, randomized controlled trial - Study protocol
Erratum in
-
Erratum to: CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: A multi-center, multi-national, randomized controlled trial - Study protocol.BMC Pregnancy Childbirth. 2016 Aug 26;16(1):249. doi: 10.1186/s12884-016-1036-3. BMC Pregnancy Childbirth. 2016. PMID: 27565557 Free PMC article. No abstract available.
Abstract
Background: Women with type 1 diabetes strive for optimal glycemic control before and during pregnancy to avoid adverse obstetric and perinatal outcomes. For most women, optimal glycemic control is challenging to achieve and maintain. The aim of this study is to determine whether the use of real-time continuous glucose monitoring (RT-CGM) will improve glycemic control in women with type 1 diabetes who are pregnant or planning pregnancy.
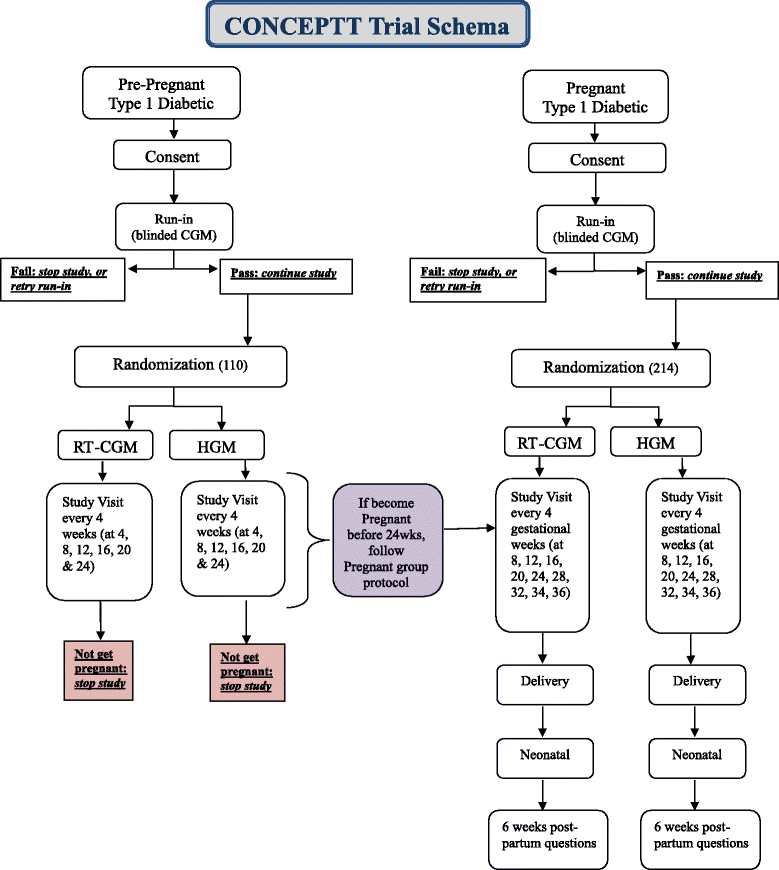
Methods/design: A multi-center, open label, randomized, controlled trial of women with type 1 diabetes who are either planning pregnancy with an HbA1c of 7.0 % to ≤10.0 % (53 to ≤ 86 mmol/mol) or are in early pregnancy (<13 weeks 6 days) with an HbA1c of 6.5 % to ≤10.0 % (48 to ≤ 86 mmol/mol). Participants will be randomized to either RT-CGM alongside conventional intermittent home glucose monitoring (HGM), or HGM alone. Eligible women will wear a CGM which does not display the glucose result for 6 days during the run-in phase. To be eligible for randomization, a minimum of 4 HGM measurements per day and a minimum of 96 hours total with 24 hours overnight (11 pm-7 am) of CGM glucose values are required. Those meeting these criteria are randomized to RT- CGM or HGM. A total of 324 women will be recruited (110 planning pregnancy, 214 pregnant). This takes into account 15 and 20 % attrition rates for the planning pregnancy and pregnant cohorts and will detect a clinically relevant 0.5 % difference between groups at 90 % power with 5 % significance. Randomization will stratify for type of insulin treatment (pump or multiple daily injections) and baseline HbA1c. Analyses will be performed according to intention to treat. The primary outcome is the change in glycemic control as measured by HbA1c from baseline to 24 weeks or conception in women planning pregnancy, and from baseline to 34 weeks gestation during pregnancy. Secondary outcomes include maternal hypoglycemia, CGM time in, above and below target (3.5-7.8 mmol/l), glucose variability measures, maternal and neonatal outcomes.
Discussion: This will be the first international multicenter randomized controlled trial to evaluate the impact of RT- CGM before and during pregnancy in women with type 1 diabetes.
Trial registration: ClinicalTrials.gov Identifier: NCT01788527 Registration Date: December 19, 2012.
Keywords: Continuous glucose monitoring; Diabetes mellitus type 1; Preconception; Pregnancy; Randomized controlled trial.
References
-
- Murphy HR, Roland JM, Skinner TC, Simmons D, Gurnell E, Morrish NJ, Soo SC, Kelly S, Lim B, Randall J, et al. Effectiveness of a Regional Prepregnancy Care Program in Women With Type 1 and Type 2 Diabetes: Benefits beyond glycemic control. Diabetes Care. 2010;33(12):2514–2520. doi: 10.2337/dc10-1113. - DOI - PMC - PubMed
-
- Yogev Y, Chen R, Ben-Haroush A, Phillip M, Jovanovic L, Hod M. Continuous glucose monitoring for the evaluation of gravid women with type 1 diabetes mellitus. Obstet Gynecol. 2003;101(4):633–638. - PubMed
-
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical