HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis
- PMID: 27430718
- PMCID: PMC4976004
- DOI: 10.4049/jimmunol.1600266
HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis
Abstract
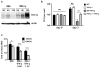
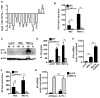
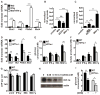
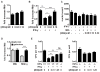
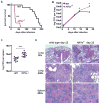
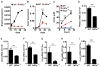
The cytokine IFN-γ coordinates macrophage activation and is essential for control of pathogens, including Mycobacterium tuberculosis However, the mechanisms by which IFN-γ controls M. tuberculosis infection are only partially understood. In this study, we show that the transcription factor hypoxia-inducible factor-1α (HIF-1α) is an essential mediator of IFN-γ-dependent control of M. tuberculosis infection both in vitro and in vivo. M. tuberculosis infection of IFN-γ-activated macrophages results in a synergistic increase in HIF-1α protein levels. This increase in HIF-1α levels is functionally important, as macrophages lacking HIF-1α are defective for IFN-γ-dependent control of infection. RNA-sequencing demonstrates that HIF-1α regulates nearly one-half of all IFN-γ-inducible genes during infection of macrophages. In particular, HIF-1α regulates production of important immune effectors, including inflammatory cytokines and chemokines, eicosanoids, and NO. In addition, we find that during infection HIF-1α coordinates a metabolic shift to aerobic glycolysis in IFN-γ-activated macrophages. We find that this enhanced glycolytic flux is crucial for IFN-γ-dependent control of infection in macrophages. Furthermore, we identify a positive feedback loop between HIF-1α and aerobic glycolysis that amplifies macrophage activation. Finally, we demonstrate that HIF-1α is crucial for control of infection in vivo as mice lacking HIF-1α in the myeloid lineage are strikingly susceptible to infection and exhibit defective production of inflammatory cytokines and microbicidal effectors. In conclusion, we have identified HIF-1α as a novel regulator of IFN-γ-dependent immunity that coordinates an immunometabolic program essential for control of M. tuberculosis infection in vitro and in vivo.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
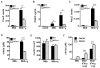
References
-
- Floyd K. World Health Organization, editor. Global Tuberculosis Report 2012. WHO Press; 2012. pp. 1–100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

