Penetration of Ceftaroline into the Epithelial Lining Fluid of Healthy Adult Subjects
- PMID: 27431215
- PMCID: PMC5038321
- DOI: 10.1128/AAC.02755-15
Penetration of Ceftaroline into the Epithelial Lining Fluid of Healthy Adult Subjects
Abstract
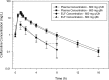
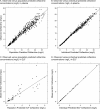
Ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, is a cephalosporin with bactericidal activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA). This study aimed to (i) evaluate ceftaroline concentrations in human plasma and epithelial lining fluid (ELF) and (ii) develop a population pharmacokinetic (PK) model for plasma and ELF to be used in PK/pharmacodynamic (PD) target attainment simulations. Ceftaroline concentrations in ELF and plasma at steady state (day 4) were measured in healthy adult subjects for two dosages: 600 mg every 12 h (q12h) and 600 mg every 8 h (q8h). Both were well tolerated with no serious adverse events. The penetration of free ceftaroline into ELF, assuming 20% protein binding in plasma and no protein binding in ELF, was ≈23%. The population PK model utilized a two-compartment model for both ceftaroline fosamil and ceftaroline. Goodness-of-fit criteria revealed the model was consistent with observed data and no systematic bias remained. At 600 mg q12h and a MIC of 1 mg/liter, 98.1% of simulated patients would be expected to achieve a target free drug concentration above the MIC (fT>MIC) in plasma of 42%, and in ELF 81.7% would be expected to achieve a target fT>MIC of 17%; at 600 mg q8h, 100% were predicted to achieve an fT>MIC in plasma of 42% and 94.7% to achieve an fT>MIC of 17% in ELF. The literature and data suggest the 600 mg q12h dose is adequate for MICs of ≤1 mg/liter. There is a need for clinical data in patients with MRSA pneumonia and data to correlate PK/PD relationships in ELF with clinical outcomes.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Flamm RK, Sader HS, Farrell DJ, Jones RN. 2012. Summary of ceftaroline activity against pathogens in the United States, 2010: report from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance program. Antimicrob Agents Chemother 56:2933–2940. doi:10.1128/AAC.00330-12. - DOI - PMC - PubMed
-
- File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley IA, Thye DA, FOCUS 1 Investigators . 2011. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66(Suppl 3):iii19–iii32. - PubMed
-
- File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2010. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, double-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 51:1395–1405. doi:10.1086/657313. - DOI - PubMed
-
- Low DE, File TM Jr, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley IA, Thye DA, FOCUS 2 Investigators . 2011. FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66(Suppl 3):iii33–iii44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

