Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity
- PMID: 27431232
- PMCID: PMC5038267
- DOI: 10.1128/AAC.00341-16
Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity
Abstract
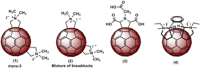
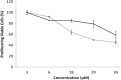
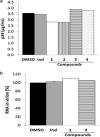
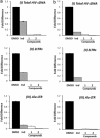
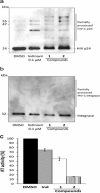
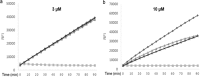
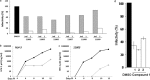
Three compounds (1, 2, and 3) previously reported to inhibit HIV-1 replication and/or in vitro activity of reverse transcriptase were studied, but only fullerene derivatives 1 and 2 showed strong antiviral activity on the replication of HIV-1 in human CD4(+) T cells. However, these compounds did not inhibit infection by single-round infection vesicular stomatitis virus glycoprotein G (VSV-G)-pseudotyped viruses, indicating no effect on the early steps of the viral life cycle. In contrast, analysis of single-round infection VSV-G-pseudotyped HIV-1 produced in the presence of compound 1 or 2 showed a complete lack of infectivity in human CD4(+) T cells, suggesting that the late stages of the HIV-1 life cycle were affected. Quantification of virion-associated viral RNA and p24 indicates that RNA packaging and viral production were unremarkable in these viruses. However, Gag and Gag-Pol processing was affected, as evidenced by immunoblot analysis with an anti-p24 antibody and the measurement of virion-associated reverse transcriptase activity, ratifying the effect of the fullerene derivatives on virion maturation of the HIV-1 life cycle. Surprisingly, fullerenes 1 and 2 did not inhibit HIV-1 protease in an in vitro assay at the doses that potently blocked viral infectivity, suggesting a protease-independent mechanism of action. Highlighting the potential therapeutic relevance of fullerene derivatives, these compounds block infection by HIV-1 resistant to protease and maturation inhibitors.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Characterization of New Cationic N,N-Dimethyl[70]fulleropyrrolidinium Iodide Derivatives as Potent HIV-1 Maturation Inhibitors.J Med Chem. 2016 Dec 22;59(24):10963-10973. doi: 10.1021/acs.jmedchem.6b00994. Epub 2016 Nov 30. J Med Chem. 2016. PMID: 28002960
-
Fullerene Derivatives Prevent Packaging of Viral Genomic RNA into HIV-1 Particles by Binding Nucleocapsid Protein.Viruses. 2021 Dec 6;13(12):2451. doi: 10.3390/v13122451. Viruses. 2021. PMID: 34960720 Free PMC article.
-
Novel Acylguanidine-Based Inhibitor of HIV-1.J Virol. 2016 Sep 29;90(20):9495-508. doi: 10.1128/JVI.01107-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27512074 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
Proteolytic events of HIV-1 replication as targets for therapeutic intervention.Curr Pharm Des. 2003;9(22):1803-15. doi: 10.2174/1381612033454478. Curr Pharm Des. 2003. PMID: 12871198 Review.
Cited by
-
Advances in Antiviral Material Development.Chempluschem. 2020 Aug 21;85(9):2105-28. doi: 10.1002/cplu.202000460. Online ahead of print. Chempluschem. 2020. PMID: 32881384 Free PMC article.
-
Synthesis of indano[60]fullerene thioketone and its application in organic solar cells.Beilstein J Org Chem. 2024 May 31;20:1270-1277. doi: 10.3762/bjoc.20.109. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38887582 Free PMC article.
-
Fullerenes in Biology and Medicine.J Mater Chem B. 2017;5(32):6523-6535. doi: 10.1039/C7TB00855D. Epub 2017 Jul 8. J Mater Chem B. 2017. PMID: 29225883 Free PMC article.
-
Nano-based approaches in the development of antiviral agents and vaccines.Life Sci. 2021 Jan 15;265:118761. doi: 10.1016/j.lfs.2020.118761. Epub 2020 Nov 12. Life Sci. 2021. PMID: 33189824 Free PMC article. Review.
-
Nanotechnology-based antiviral therapeutics.Drug Deliv Transl Res. 2021 Jun;11(3):748-787. doi: 10.1007/s13346-020-00818-0. Drug Deliv Transl Res. 2021. PMID: 32748035 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

