BAT117213: Ileal bile acid transporter (IBAT) inhibition as a treatment for pruritus in primary biliary cirrhosis: study protocol for a randomised controlled trial
- PMID: 27431238
- PMCID: PMC4950723
- DOI: 10.1186/s12876-016-0481-9
BAT117213: Ileal bile acid transporter (IBAT) inhibition as a treatment for pruritus in primary biliary cirrhosis: study protocol for a randomised controlled trial
Abstract
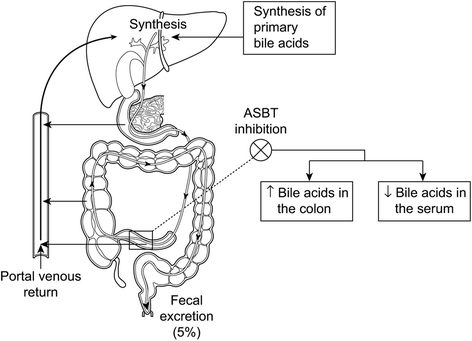
Background: Pruritus (itch) is a symptom commonly experienced by patients with cholestatic liver diseases such as primary biliary cholangitis (PBC, previously referred to as primary biliary cirrhosis). Bile acids (BAs) have been proposed as potential pruritogens in PBC. The ileal bile acid transporter (IBAT) protein expressed in the distal ileum plays a key role in the enterohepatic circulation of BAs. Pharmacological inhibition of IBAT with GSK2330672 may reduce BA levels in the systemic circulation and improve pruritus.
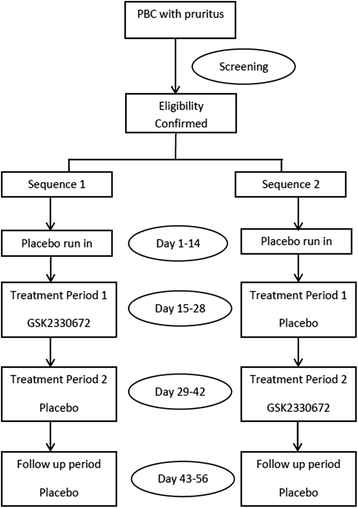
Methods: This clinical study (BAT117213 study) is sponsored by GlaxoSmithKline (GSK) with associated exploratory studies supported by the National Institute for Health Research (NIHR). It is a phase 2a, multi-centre, randomised, double bind, placebo controlled, cross-over trial for PBC patients with pruritus. The primary objective is to investigate the safety and tolerability of repeat doses of GSK2330672, and explore whether GSK2330672 administration for 14 days improves pruritus compared with placebo. The key outcomes include improvement in pruritus scores evaluated on a numerical rating scale and other PBC symptoms in an electronic diary completed twice daily by the patients. The secondary outcomes include the evaluation of the effect of GSK2330672 on total serum bile acid (BA) concentrations, serum markers of BA synthesis and steady-state pharmacokinetics of ursodeoxycholic acid (UDCA).
Discussion: BAT117213 study is the first randomised controlled crossover trial of ileal bile acid transporter inhibitor, a novel class of drug to treat pruritus in PBC. The main strengths of the trial are utility of a novel, study specific, electronic symptom diary as patient reported outcome to measure the treatment response objectively and the crossover design that allows estimating the treatment effect in a smaller number of patients. The outcome of this trial will inform the trial design of future development phase of the IBAT inhibitor drug. The trial will also provide opportunity to conduct metabonomic and gut microbiome studies as explorative and mechanistic research in patients with cholestatic pruritus.
Trial registration: EudraCT number: 2012-005531-84, ClinicalTrials.gov Identifier: NCT01899703 , registered on 3(rd) July 2013.
Keywords: IBAT; Ileal bile acid transporter; PBC; Primary biliary cholangitis; Pruritus.
Figures
Similar articles
-
Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study.Lancet. 2017 Mar 18;389(10074):1114-1123. doi: 10.1016/S0140-6736(17)30319-7. Epub 2017 Feb 8. Lancet. 2017. PMID: 28187915 Clinical Trial.
-
Autotaxin, bile acid profile and effect of ileal bile acid transporter inhibition in primary biliary cholangitis patients with pruritus.Liver Int. 2019 May;39(5):967-975. doi: 10.1111/liv.14069. Epub 2019 Feb 25. Liver Int. 2019. PMID: 30735608
-
Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis.Sci Rep. 2018 Apr 27;8(1):6658. doi: 10.1038/s41598-018-25214-0. Sci Rep. 2018. PMID: 29704003 Free PMC article. Clinical Trial.
-
Sodium/bile acid co-transporter inhibitors currently in preclinical or early clinical development for the treatment of primary biliary cholangitis.Expert Opin Investig Drugs. 2024 May;33(5):485-495. doi: 10.1080/13543784.2024.2343789. Epub 2024 Apr 24. Expert Opin Investig Drugs. 2024. PMID: 38613839 Review.
-
Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review.Int J Mol Sci. 2025 Feb 22;26(5):1883. doi: 10.3390/ijms26051883. Int J Mol Sci. 2025. PMID: 40076514 Free PMC article. Review.
Cited by
-
Treatment of Pruritus Secondary to Liver Disease.Curr Gastroenterol Rep. 2019 Jul 31;21(9):48. doi: 10.1007/s11894-019-0713-6. Curr Gastroenterol Rep. 2019. PMID: 31367993 Review.
-
Primary biliary cholangitis: a summary of pathogenesis and therapies.Ann Gastroenterol. 2025 Mar-Apr;38(2):121-132. doi: 10.20524/aog.2025.0953. Epub 2025 Feb 28. Ann Gastroenterol. 2025. PMID: 40124425 Free PMC article. Review.
-
Development and adaptation of patient-reported outcome measures for patients who experience itch associated with primary biliary cholangitis.J Patient Rep Outcomes. 2019 Jan 15;3(1):2. doi: 10.1186/s41687-018-0090-1. J Patient Rep Outcomes. 2019. PMID: 30645706 Free PMC article.
-
[Treatment of chronic pruritus-what is new?].Hautarzt. 2018 Aug;69(8):641-646. doi: 10.1007/s00105-018-4221-7. Hautarzt. 2018. PMID: 29931387 Review. German.
-
Preoperative Nasobiliary Drainage as a Predictor of Response Before Surgical Fistula Creation in Joubert Syndrome With Refractory Cholestatic Pruritis.ACG Case Rep J. 2023 Oct 3;10(10):e01171. doi: 10.14309/crj.0000000000001171. eCollection 2023 Oct. ACG Case Rep J. 2023. PMID: 37799488 Free PMC article.
References
-
- Hegade VS, Mells GF, Beuers U, et al. Patient Experience and Characteristics of Cholestatic Pruritus in the UK-PBC Research Cohort. Hepatology. 2014;60:339A–69. doi: 10.1002/hep.27496. - DOI
-
- Hegade VS, Mells GF, Lammert C, et al. A Comparative Study of Pruritus in PBC cohorts from UK, USA and Italy. J Hepatol. 2015;62:S785. doi: 10.1016/S0168-8278(15)31349-0. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

