PSA nadir as a predictive factor for biochemical disease-free survival and overall survival following whole-gland salvage HIFU following radiotherapy failure
- PMID: 27431499
- PMCID: PMC4983180
- DOI: 10.1038/pcan.2016.23
PSA nadir as a predictive factor for biochemical disease-free survival and overall survival following whole-gland salvage HIFU following radiotherapy failure
Abstract
Background: Treatment options for radio-recurrent prostate cancer are either androgen-deprivation therapy or salvage prostatectomy. Whole-gland high-intensity focussed ultrasound (HIFU) might have a role in this setting.
Methods: An independent HIFU registry collated consecutive cases of HIFU. Between 2005 and 2012, we identified 50 men who underwent whole-gland HIFU following histological confirmation of localised disease following prior external beam radiotherapy (2005-2012). No upper threshold was applied for risk category, PSA or Gleason grade either at presentation or at the time of failure. Progression was defined as a composite with biochemical failure (Phoenix criteria (PSA>nadir+2 ng ml(-1))), start of systemic therapies or metastases.
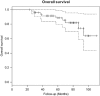
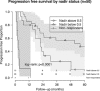
Results: Median age (interquartile range (IQR)), pretreatment PSA (IQR) and Gleason score (range) were 68 years (64-72), 5.9 ng ml(-1) (2.2-11.3) and 7 (6-9), respectively. Median follow-up was 64 months (49-84). In all, 24/50 (48%) avoided androgen-deprivation therapies. Also, a total of 28/50 (56%) achieved a PSA nadir <0.5 ng ml(-1), 15/50 (30%) had a nadir ⩾0.5 ng ml(-1) and 7/50 (14%) did not nadir (PSA non-responders). Actuarial 1, 3 and 5-year progression-free survival (PFS) was 72, 40 and 31%, respectively. Actuarial 1, 3 and 5-year overall survival (OS) was 100, 94 and 87%, respectively. When comparing patients with PSA nadir <0.5 ng ml(-1), nadir ⩾0.5 and non-responders, a statistically significant difference in PFS was seen (P<0.0001). Three-year PFS in each group was 57, 20 and 0%, respectively. Five-year OS was 96, 100 and 38%, respectively. Early in the learning curve, between 2005 and 2007, 3/50 (6%) developed a fistula. Intervention for bladder outlet obstruction was needed in 27/50 (54%). Patient-reported outcome measure questionnaires showed incontinence (any pad-use) as 8/26 (31%).
Conclusions: In our series of high-risk patients, in whom 30-50% may have micro-metastases, disease control rates were promising in PSA responders, however, with significant morbidity. Additionally, post-HIFU PSA nadir appears to be an important predictor for both progression and survival. Further research on focal salvage ablation in order to reduce toxicity while retaining disease control rates is required.
Conflict of interest statement
H.U. Ahmed would like to acknowledge funding from the Medical Research Council (UK), the Pelican Cancer Foundation Charity, Prostate Cancer UK, St Peters Trust Charity, Prostate Cancer Research Centre the Wellcome Trust, National Institute of Health Research-Health Technology Assessment Programme, and the US National Institute of Health-National Cancer Institute. H.U. Ahmed also receives funding from USHIFU, Trod Medical and Sophiris Biocorp for clinical trials. Mark Emberton has been awarded National Institute of Health Research (NIHR) Senior Investigator status. He receives research support from the UCLH/UCL NIHR Biomedical Research Centre. Mark Emberton receives funding from USHIFU, Trod Medical and Sophiris Biocorp for clinical trials and is a consultant for Steba Biotech and USHIFU. He also receives funding from the Medical Research Council (UK), the Pelican Cancer Foundation Charity, Prostate Cancer UK, St Peters Trust Charity, Prostate Cancer Research Centre the Wellcome Trust, National Institute of Health Research-Health Technology Assessment Programme, and the US National Institute of Health-National Cancer Institute. T.T. Shah would like to acknowledge funding from the St Peters Trust for clinical research and has received funding for conference attendance from Astellis, Ferring, and Galil medical.
Figures
Similar articles
-
High-intensity Focused Ultrasound (HIFU) as salvage therapy for radio-recurrent prostate cancer: predictors of disease response.Int Braz J Urol. 2018 Mar-Apr;44(2):248-257. doi: 10.1590/S1677-5538.IBJU.2017.0025. Int Braz J Urol. 2018. PMID: 29211405 Free PMC article.
-
Whole-gland salvage high-intensity focused ultrasound therapy for localized prostate cancer recurrence after external beam radiation therapy.Cancer. 2012 Jun 15;118(12):3071-8. doi: 10.1002/cncr.26631. Epub 2011 Nov 9. Cancer. 2012. PMID: 22071795
-
Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients.Eur Urol. 2014 May;65(5):907-14. doi: 10.1016/j.eururo.2013.04.039. Epub 2013 Apr 30. Eur Urol. 2014. PMID: 23669165 Clinical Trial.
-
High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience.Urology. 2004 Feb;63(2):297-300. doi: 10.1016/j.urology.2003.09.020. Urology. 2004. PMID: 14972475 Review.
-
Management of localised prostate cancer: watchful waiting, surgery or radiation therapy, depending on the natural course, which is often relatively slow.Prescrire Int. 2012 Oct;21(131):242-8. Prescrire Int. 2012. PMID: 23185849 Review.
Cited by
-
Local salvage therapies in patients with radio-recurrent prostate cancer following external beam radiotherapy: a systematic review and meta-analysis.Prostate Cancer Prostatic Dis. 2025 Sep;28(3):578-591. doi: 10.1038/s41391-024-00883-3. Epub 2024 Sep 2. Prostate Cancer Prostatic Dis. 2025. PMID: 39223232
-
Diagnostic value of multiparametric MRI in detecting residual or recurrent prostate cancer after high-intensity focused ultrasound.Prostate Cancer Prostatic Dis. 2023 Jun;26(2):360-366. doi: 10.1038/s41391-022-00531-8. Epub 2022 May 28. Prostate Cancer Prostatic Dis. 2023. PMID: 35643729
-
Oncologic outcome of salvage high-intensity focused ultrasound (HIFU) in radiorecurrent prostate cancer. A systematic review.Acta Biomed. 2021 Sep 2;92(4):e2021191. doi: 10.23750/abm.v92i3.11475. Acta Biomed. 2021. PMID: 34487074 Free PMC article.
-
Functional Outcomes after Local Salvage Therapies for Radiation-Recurrent Prostate Cancer Patients: A Systematic Review.Cancers (Basel). 2021 Jan 11;13(2):244. doi: 10.3390/cancers13020244. Cancers (Basel). 2021. PMID: 33440752 Free PMC article. Review.
-
Salvage Therapy Options for Local Prostate Cancer Recurrence After Primary Radiotherapy: a Literature Review.Curr Urol Rep. 2017 Aug;18(8):63. doi: 10.1007/s11934-017-0709-4. Curr Urol Rep. 2017. PMID: 28688020 Review.
References
-
- UK CR. Prostate Cancer Statistics, 2011. Cancer Research UK, http://www.cancerresearchuk.org/health-professional/cancer-statistics/st.... Accessed February 2016.
-
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol 2004; 171: 1132–1136. - PubMed
-
- Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR, Cancer of the Prostate Strategic Urological Research Endeavor. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 2008; 112: 307–314. - PubMed
-
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373: 301–308. - PubMed
-
- Sharifi N, Dahut WL, Steinberg SM, Figg WD, Tarassoff C, Arlen P et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int 2005; 96: 985–989. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous