Genetic Abrogation of Adenosine A3 Receptor Prevents Uninephrectomy and High Salt-Induced Hypertension
- PMID: 27431647
- PMCID: PMC5015411
- DOI: 10.1161/JAHA.116.003868
Genetic Abrogation of Adenosine A3 Receptor Prevents Uninephrectomy and High Salt-Induced Hypertension
Abstract
Background: Early-life reduction in nephron number (uninephrectomy [UNX]) and chronic high salt (HS) intake increase the risk of hypertension and chronic kidney disease. Adenosine signaling via its different receptors has been implicated in modulating renal, cardiovascular, and metabolic functions as well as inflammatory processes; however, the specific role of the A3 receptor in cardiovascular diseases is not clear. In this study, gene-modified mice were used to investigate the hypothesis that lack of A3 signaling prevents the development of hypertension and attenuates renal and cardiovascular injuries following UNX in combination with HS (UNX-HS) in mice.
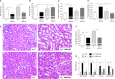
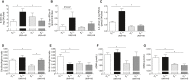
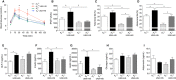
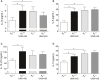
Methods and results: Wild-type (A3 (+/+)) mice subjected to UNX-HS developed hypertension compared with controls (mean arterial pressure 106±3 versus 82±3 mm Hg; P<0.05) and displayed an impaired metabolic phenotype (eg, increased adiposity, reduced glucose tolerance, hyperinsulinemia). These changes were associated with both cardiac hypertrophy and fibrosis together with renal injuries and proteinuria. All of these pathological hallmarks were significantly attenuated in the A3 (-/-) mice. Mechanistically, absence of A3 receptors protected from UNX-HS-associated increase in renal NADPH oxidase activity and Nox2 expression. In addition, circulating cytokines including interleukins 1β, 6, 12, and 10 were increased in A3 (+/+) following UNX-HS, but these cytokines were already elevated in naïve A3 (-/-) mice and did not change following UNX-HS.
Conclusions: Reduction in nephron number combined with chronic HS intake is associated with oxidative stress, chronic inflammation, and development of hypertension in mice. Absence of adenosine A3 receptor signaling was strongly protective in this novel mouse model of renal and cardiovascular disease.
Keywords: NADPH oxidase; adenosine receptor; cardiovascular disease; high salt diet; inflammation; kidney; nephron number; uninephrectomy.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures




Similar articles
-
Uninephrectomy in young age or chronic salt loading causes salt-sensitive hypertension in adult rats.Hypertension. 2007 Jun;49(6):1342-50. doi: 10.1161/HYPERTENSIONAHA.107.087213. Epub 2007 Apr 16. Hypertension. 2007. PMID: 17438306
-
L-arginine or tempol supplementation improves renal and cardiovascular function in rats with reduced renal mass and chronic high salt intake.Acta Physiol (Oxf). 2013 Apr;207(4):732-41. doi: 10.1111/apha.12079. Epub 2013 Feb 25. Acta Physiol (Oxf). 2013. PMID: 23387940
-
Renal denervation attenuates hypertension and renal dysfunction in a model of cardiovascular and renal disease, which is associated with reduced NADPH and xanthine oxidase activity.Redox Biol. 2017 Oct;13:522-527. doi: 10.1016/j.redox.2017.06.013. Epub 2017 Jul 5. Redox Biol. 2017. PMID: 28734244 Free PMC article.
-
Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension.Adv Exp Med Biol. 2017;956:61-84. doi: 10.1007/5584_2016_147. Adv Exp Med Biol. 2017. PMID: 27757935 Review.
-
[Sodium, hypertension, chronic kidney diseases, and public health].Nephrol Ther. 2018 Apr;14 Suppl 1:S93-S98. doi: 10.1016/j.nephro.2018.02.002. Nephrol Ther. 2018. PMID: 29606270 Review. French.
Cited by
-
Mechanisms in hypertension and target organ damage: Is the role of the thymus key? (Review).Int J Mol Med. 2018 Jul;42(1):3-12. doi: 10.3892/ijmm.2018.3605. Epub 2018 Mar 30. Int J Mol Med. 2018. PMID: 29620247 Free PMC article. Review.
-
Stellate ganglion irradiation alleviates airway inflammation in asthmatic mice via activating SIRT1 signaling pathway.Sci Rep. 2025 Aug 1;15(1):28092. doi: 10.1038/s41598-025-12901-y. Sci Rep. 2025. PMID: 40750982 Free PMC article.
-
Adenosine receptors as emerging therapeutic targets for diabetic kidney disease.Kidney Res Clin Pract. 2022 Sep;41(Suppl 2):S74-S88. doi: 10.23876/j.krcp.22.011. Epub 2022 Aug 22. Kidney Res Clin Pract. 2022. PMID: 36239063 Free PMC article.
-
Pathological overproduction: the bad side of adenosine.Br J Pharmacol. 2017 Jul;174(13):1945-1960. doi: 10.1111/bph.13763. Epub 2017 Mar 31. Br J Pharmacol. 2017. PMID: 28252203 Free PMC article. Review.
-
Potassium-Alkali-Enriched Diet, Hypertension, and Proteinuria following Uninephrectomy.J Am Soc Nephrol. 2024 Oct 1;35(10):1330-1350. doi: 10.1681/ASN.0000000000000420. Epub 2024 Jun 24. J Am Soc Nephrol. 2024. PMID: 38913441
References
-
- Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney‐associated outcomes—a global concern. Nat Rev Nephrol. 2015;11:135–149. - PubMed
-
- Carlstrom M, Sallstrom J, Skott O, Larsson E, Persson AE. Uninephrectomy in young age or chronic salt loading causes salt‐sensitive hypertension in adult rats. Hypertension. 2007;49:1342–1350. - PubMed
-
- Moritz KM, Wintour EM, Dodic M. Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension. 2002;39:1071–1076. - PubMed
-
- Woods LL, Weeks DA, Rasch R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension. 2001;38:337–342. - PubMed
-
- Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt‐induced hypertension. Cardiovasc Res. 2011;89:574–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

