MicroRNA-183 suppresses cancer stem-like cell properties in EBV-associated nasopharyngeal carcinoma
- PMID: 27431799
- PMCID: PMC4950376
- DOI: 10.1186/s12885-016-2525-5
MicroRNA-183 suppresses cancer stem-like cell properties in EBV-associated nasopharyngeal carcinoma
Abstract
Background: Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-associated epithelial malignancy that exhibits distinct geographical and ethnic prevalence. Although the contemporary therapeutic approach of radio-/chemotherapy provides excellent results for patients with early-stage disease, it is far from satisfactory for those with disease remission and distant metastasis. Promising therapeutic strategies for advanced and relapsed NPC are still lacking. We recently identified and characterized a cancer stem-like cell (CSC) subpopulation in NPC that appeared to play an important role in tumor progression. Microarray analysis revealed downregulation of several stemness-inhibiting miRNAs in these CSC cells. Among these miRNAs, miR-96 and miR-183 showed the highest fold change and were selected to elucidate their role in repressing NPC CSC properties.
Methods: MiR-96 and miR-183 expression in NPC CSCs was detected by qRT-PCR. Transient and stable transfection was performed in EBV-positive NPC C666-1 cells to examine the effects of ectopic expression of miR-96 and miR-183 on repressing cell growth and CSC properties. Anchorage-dependent (colony formation) and anchorage-independent (tumor sphere formation) growths of these miR-96 and miR-183 expressing cells were determined. Expression of multiple CSC markers and related molecules were accessed by flow cytometry and Western blotting. The tumorigenicity of the stable miR-96- and miR-183-transfected NPC cells was examined in an in vivo nude mice model.
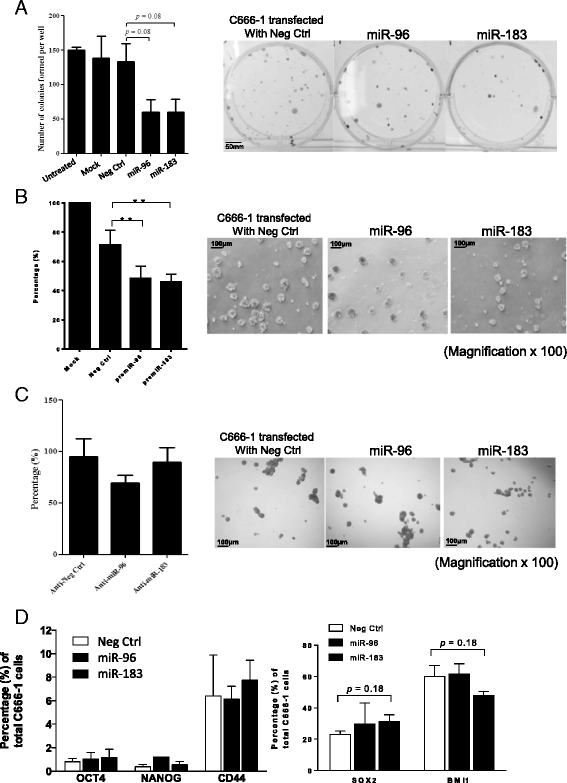
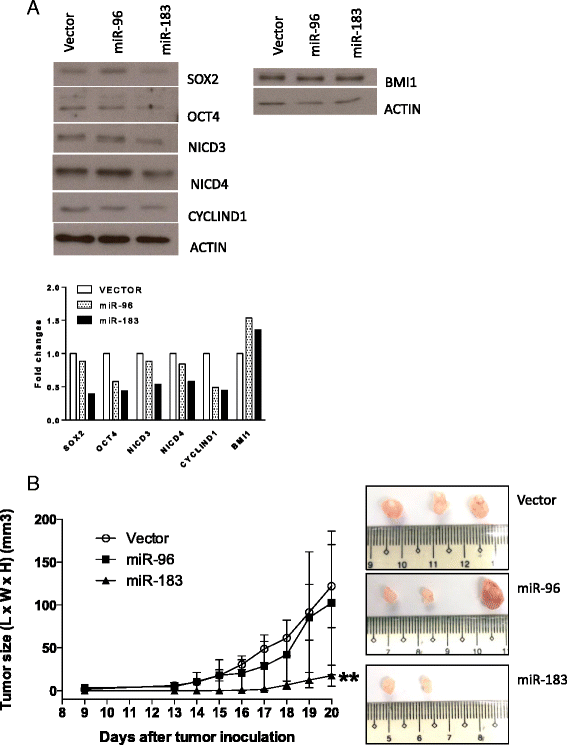
Results: Downregulation of miR-96 and miR-183 was confirmed in NPC spheroids. Using transient or stable transfection, we showed that ectopic expression of miR-96 and miR-183 suppressed cell growth and tumor sphere formation in NPC. Reduced NICD3 and NICD4 in miR-96- and miR-183-expressing NPC cells suggests the involvement of the NOTCH signaling pathway in their tumor suppressive function. Finally, we showed that the tumorigenicity of cells stably expressing miR-183 was significantly inhibited in the in vivo nude mice model.
Conclusions: miR-183 is a tumor-suppressive miRNA in EBV-associated NPC. Its abilities to suppress CSC properties in vitro and effectively reduce tumor growth in vivo shed light on its role as a potential therapeutic target.
Keywords: Cancer stem-like cells; Epstein-Barr virus; NOTCH; Nasopharyngeal carcinoma; microRNA.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

