The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood-brain barrier in vitro
- PMID: 27431938
- PMCID: PMC5042357
- DOI: 10.1016/j.brainres.2016.07.026
The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood-brain barrier in vitro
Abstract
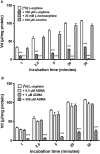
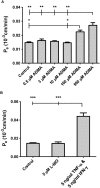
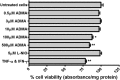
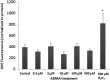
The blood-brain barrier (BBB) is a biological firewall that carefully regulates the cerebral microenvironment by acting as a physical, metabolic and transport barrier. This selectively permeable interface was modelled using the immortalised human cerebral microvascular endothelial cell line (hCMEC/D3) to investigate interactions with the cationic amino acid (CAA) L-arginine, the precursor for nitric oxide (NO), and with asymmetric dimethylarginine (ADMA), an endogenously derived analogue of L-arginine that potently inhibits NO production. The transport mechanisms utilised by L-arginine are known but they are not fully understood for ADMA, particularly at the BBB. This is of clinical significance giving the emerging role of ADMA in many brain and cerebrovascular diseases and its potential as a therapeutic target. We discovered that high concentrations of ADMA could induce endothelial dysfunction in the hCMEC/D3s BBB permeability model, leading to an increase in paracellular permeability to the paracellular marker FITC-dextran (40kDa). We also investigated interactions of ADMA with a variety of transport mechanisms, comparing the data with L-arginine interactions. Both molecules are able to utilise the CAA transport system y(+). Furthermore, the expression of CAT-1, the best known protein from this group, was confirmed in the hCMEC/D3s. It is likely that influx systems, such as y(+)L and b(0,+), have an important physiological role in ADMA transport at the BBB. These data are not only important with regards to the brain, but apply to other microvascular endothelia where ADMA is a major area of investigation.
Keywords: Cationic amino acid transporter 1; Cerebral microvasculature; Guanosine triphosphatases (GTPases); Nitric oxide.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Saturation kinetics and specificity of transporters for L-arginine and asymmetric dimethylarginine (ADMA) at the blood-brain and blood-CSF barriers.PLoS One. 2025 Jun 11;20(6):e0320034. doi: 10.1371/journal.pone.0320034. eCollection 2025. PLoS One. 2025. PMID: 40498714 Free PMC article.
-
L-Arginine and asymmetric dimethylarginine (ADMA) transport across the mouse blood-brain and blood-CSF barriers: Evidence of saturable transport at both interfaces and CNS to blood efflux.PLoS One. 2024 Oct 24;19(10):e0305318. doi: 10.1371/journal.pone.0305318. eCollection 2024. PLoS One. 2024. PMID: 39446890 Free PMC article.
-
Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1).J Mol Cell Cardiol. 2012 Sep;53(3):392-400. doi: 10.1016/j.yjmcc.2012.06.002. Epub 2012 Jun 15. J Mol Cell Cardiol. 2012. PMID: 22705145
-
The impact of asymmetric dimethylarginine (ADAMA), the endogenous nitric oxide (NO) synthase inhibitor, to the pathogenesis of gastric mucosal damage.Curr Pharm Des. 2013;19(1):90-7. doi: 10.2174/13816128130113. Curr Pharm Des. 2013. PMID: 22950506 Review.
-
Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L-arginine paradox" and acts as a novel cardiovascular risk factor.J Nutr. 2004 Oct;134(10 Suppl):2842S-2847S; discussion 2853S. doi: 10.1093/jn/134.10.2842S. J Nutr. 2004. PMID: 15465797 Review.
Cited by
-
Common epilepsy variants from the general population are not associated with epilepsy among individuals with tuberous sclerosis complex.Am J Med Genet A. 2024 Jun;194(6):e63569. doi: 10.1002/ajmg.a.63569. Epub 2024 Feb 17. Am J Med Genet A. 2024. PMID: 38366765 Free PMC article.
-
Identification of transport systems involved in eflornithine delivery across the blood-brain barrier.Front Drug Deliv. 2023 May 23;3:1113493. doi: 10.3389/fddev.2023.1113493. Front Drug Deliv. 2023. PMID: 38482132 Free PMC article.
-
Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body.Biomolecules. 2022 Mar 25;12(4):501. doi: 10.3390/biom12040501. Biomolecules. 2022. PMID: 35454090 Free PMC article. Review.
-
Region-specific blood-brain barrier transporter changes leads to increased sensitivity to amisulpride in Alzheimer's disease.Fluids Barriers CNS. 2019 Dec 17;16(1):38. doi: 10.1186/s12987-019-0158-1. Fluids Barriers CNS. 2019. PMID: 31842924 Free PMC article.
-
New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease.Int J Mol Sci. 2019 Jan 7;20(1):187. doi: 10.3390/ijms20010187. Int J Mol Sci. 2019. PMID: 30621010 Free PMC article. Review.
References
-
- Abdelmagid S.A., Rickard J.A., Mcdonald W.J., Thomas L.N., Too C.K. CAT- 1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J. Cell. Biochem. 2011;112:1084–1092. - PubMed
-
- Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. - PubMed
-
- Arlt S., Schulze F., Eichenlaub M., Maas R., Lehmbeck J.T., Schwedhelm E., Jahn H., Boger R.H. Asymmetrical dimethylarginine is increased in plasma and decreased in cerebrospinal fluid of patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2008;26:58–64. - PubMed
-
- Battisti S., Valente D., Albonici L., Bei R., Modesti A., Palumbo C. Nutritional stress and arginine auxotrophy confer high sensitivity to chloroquine toxicity in mesothelioma cells. Am. J. Respir. Cell Mol. Biol. 2012;46:498–506. - PubMed
-
- Boger R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003;59:824–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

