Time-dependent PPARγ Modulation of HIF-1α Signaling in Hypoxic Pulmonary Artery Smooth Muscle Cells
- PMID: 27432037
- PMCID: PMC5483378
- DOI: 10.1016/j.amjms.2016.03.019
Time-dependent PPARγ Modulation of HIF-1α Signaling in Hypoxic Pulmonary Artery Smooth Muscle Cells
Abstract
Background: Pathogenesis of pulmonary hypertension is complex and involves activation of the transcription factor, hypoxia-inducible factor-1 (HIF-1) that shifts cellular metabolism from aerobic respiration to glycolysis, in part, by increasing the expression of its downstream target pyruvate dehydrogenase kinase-1 (PDK-1), thereby promoting a proliferative, apoptosis-resistant phenotype in pulmonary vascular cells. Activation of the nuclear hormone transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ), attenuates pulmonary hypertension and pulmonary artery smooth muscle cell (PASMC) proliferation. In the current study, we determined whether PPARγ inhibits HIF-1α and PDK-1 expression in human PASMCs.
Methods: HPASMCs were exposed to normoxia (21% O2) or hypoxia (1% O2) for 2-72 hours ± treatment with the PPARγ-ligand, rosiglitazone (RSG, 10μM).
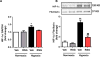
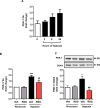
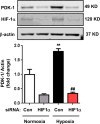
Results: Compared to normoxia, HIF-1α mRNA levels were elevated in HPASMC at 2 hours hypoxia and reduced to baseline levels by 24-72 hours. HIF-1α protein levels increased following 4 and 8 hours of hypoxia and returned to baseline levels by 24 and 72 hours. PDK-1 protein levels increased following 24 hours hypoxia and remained elevated by 72 hours. RSG treatment at the onset of hypoxia attenuated HIF-1α protein and PDK-1 mRNA and protein levels at 4, 8 and 24 hours of hypoxia, respectively. However, RSG treatment during final 24 hours of 72-hour hypoxia, an intervention that inhibits HPASMC proliferation, failed to prevent hypoxia-induced PDK-1 expression.
Conclusion: Hypoxia causes transient activation of HPASMC HIF-1α that is attenuated by RSG treatment initiated at hypoxia onset. These findings provide novel evidence that PPARγ modulates fundamental and acute cellular responses to hypoxia through both HIF-1-dependent and HIF-1-independent mechanisms.
Keywords: HIF-1α; PDK-1; PPARγ; Pulmonary hypertension; Vascular smooth muscle cell.
Published by Elsevier Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Peroxisome proliferator-activated receptor-γ enhances human pulmonary artery smooth muscle cell apoptosis through microRNA-21 and programmed cell death 4.Am J Physiol Lung Cell Mol Physiol. 2017 Aug 1;313(2):L371-L383. doi: 10.1152/ajplung.00532.2016. Epub 2017 May 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28522568 Free PMC article.
-
Peroxisome proliferator-activated receptor γ inhibits pulmonary hypertension targeting store-operated calcium entry.J Mol Med (Berl). 2015 Mar;93(3):327-42. doi: 10.1007/s00109-014-1216-4. Epub 2014 Nov 14. J Mol Med (Berl). 2015. PMID: 25391250 Free PMC article.
-
PPARγ Ligands Attenuate Hypoxia-Induced Proliferation in Human Pulmonary Artery Smooth Muscle Cells through Modulation of MicroRNA-21.PLoS One. 2015 Jul 24;10(7):e0133391. doi: 10.1371/journal.pone.0133391. eCollection 2015. PLoS One. 2015. PMID: 26208095 Free PMC article.
-
Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer.Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570-8. doi: 10.1152/ajpheart.01324.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083891 Review.
-
Expression and regulation of HIF-1a in hypoxic pulmonary hypertension: Focus on pathological mechanism and Pharmacological Treatment.Int J Med Sci. 2024 Jan 1;21(1):45-60. doi: 10.7150/ijms.88216. eCollection 2024. Int J Med Sci. 2024. PMID: 38164358 Free PMC article. Review.
Cited by
-
Hypoxia-inducible factor signaling in pulmonary hypertension.J Clin Invest. 2020 Nov 2;130(11):5638-5651. doi: 10.1172/JCI137558. J Clin Invest. 2020. PMID: 32881714 Free PMC article. Review.
-
CD146-HIF-1α hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension.Nat Commun. 2019 Aug 7;10(1):3551. doi: 10.1038/s41467-019-11500-6. Nat Commun. 2019. PMID: 31391533 Free PMC article.
-
Alcohol-Induced Glycolytic Shift in Alveolar Macrophages Is Mediated by Hypoxia-Inducible Factor-1 Alpha.Front Immunol. 2022 May 11;13:865492. doi: 10.3389/fimmu.2022.865492. eCollection 2022. Front Immunol. 2022. PMID: 35634337 Free PMC article.
-
Exercise retards ongoing adipose tissue fibrosis in diet-induced obese mice.Endocr Connect. 2021 Mar;10(3):325-335. doi: 10.1530/EC-20-0643. Endocr Connect. 2021. PMID: 33617465 Free PMC article.
-
BCR::ABL1 expression in chronic myeloid leukemia cells in low oxygen is regulated by glutamine via CD36-mediated fatty acid uptake.Cancer Cell Int. 2025 May 14;25(1):176. doi: 10.1186/s12935-025-03805-y. Cancer Cell Int. 2025. PMID: 40369538 Free PMC article.
References
-
- Rabinovitch M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:447–58. - PubMed
-
- Ameshima S, Golpon H, Cool CD, et al. Peroxisome proliferator-activated receptor gamma (ppargamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

