The role of formyl peptide receptor 1 (FPR1) in neuroblastoma tumorigenesis
- PMID: 27432059
- PMCID: PMC4950242
- DOI: 10.1186/s12885-016-2545-1
The role of formyl peptide receptor 1 (FPR1) in neuroblastoma tumorigenesis
Abstract
Background: Formyl peptide receptor 1 (FPR1) is a G protein-coupled receptor mainly expressed by the cells of myeloid origin, where it mediates the innate immune response to bacterial formylated peptides. High expression of FPR1 has been detected in various cancers but the function of FPR1 in tumorigenesis is poorly understood.
Methods: Expression of FPR1 in neuroblastoma cell lines and primary tumors was studied using RT-PCR, western blotting, immunofluorescence and immunohistochemistry. Calcium mobilization assays and western blots with phospho-specific antibodies were used to assess the functional activity of FPR1 in neuroblastoma. The tumorigenic capacity of FPR1 was assessed by xenografting of neuroblastoma cells expressing inducible FPR1 shRNA, FPR1 cDNA or control shRNA in nude mice.
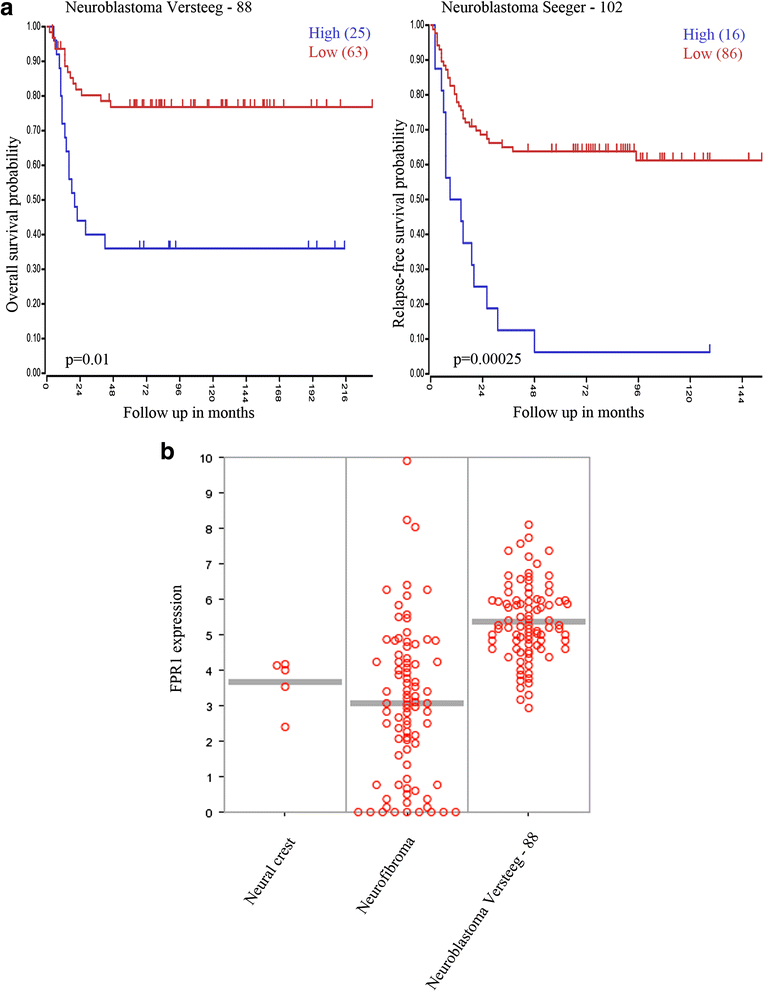
Results: FPR1 is expressed in neuroblastoma primary tumors and cell lines. High expression of FPR1 corresponds with high-risk disease and poor patient survival. Stimulation of FPR1 in neuroblastoma cells using fMLP, a selective FPR1 agonist, induced intracellular calcium mobilization and activation of MAPK/Erk, PI3K/Akt and P38-MAPK signal transduction pathways that were inhibited by using Cyclosporin H, a selective receptor antagonist for FPR1. shRNA knock-down of FPR1 in neuroblastoma cells conferred a delayed xenograft tumor development in nude mice, whereas an ectopic overexpression of FPR1 promoted augmented tumorigenesis in nude mice.
Conclusion: Our data demonstrate that FPR1 is involved in neuroblastoma development and could represent a therapy option for the treatment of neuroblastoma.
Keywords: FPR1; Formyl peptide receptor 1; Inflammation; Neuroblastoma.
Figures




Similar articles
-
Honokiol suppresses formyl peptide-induced human neutrophil activation by blocking formyl peptide receptor 1.Sci Rep. 2017 Jul 27;7(1):6718. doi: 10.1038/s41598-017-07131-w. Sci Rep. 2017. PMID: 28751674 Free PMC article.
-
Promotion of formyl peptide receptor 1-mediated neutrophil chemotactic migration by antimicrobial peptides isolated from the centipede Scolopendra subspinipes mutilans.BMB Rep. 2016 Sep;49(9):520-5. doi: 10.5483/bmbrep.2016.49.9.098. BMB Rep. 2016. PMID: 27502013 Free PMC article.
-
PEST-containing nuclear protein mediates the proliferation, migration, and invasion of human neuroblastoma cells through MAPK and PI3K/AKT/mTOR signaling pathways.BMC Cancer. 2018 May 2;18(1):499. doi: 10.1186/s12885-018-4391-9. BMC Cancer. 2018. PMID: 29716528 Free PMC article.
-
Antagonism of human formyl peptide receptor 1 with natural compounds and their synthetic derivatives.Int Immunopharmacol. 2016 Aug;37:43-58. doi: 10.1016/j.intimp.2015.08.036. Epub 2015 Sep 15. Int Immunopharmacol. 2016. PMID: 26382576 Free PMC article. Review.
-
G protein-coupled receptor FPR1 as a pharmacologic target in inflammation and human glioblastoma.Int Immunopharmacol. 2012 Nov;14(3):283-8. doi: 10.1016/j.intimp.2012.07.015. Epub 2012 Aug 2. Int Immunopharmacol. 2012. PMID: 22863814 Free PMC article. Review.
Cited by
-
Therapeutic Strategies Targeting Urokinase and Its Receptor in Cancer.Cancers (Basel). 2022 Jan 19;14(3):498. doi: 10.3390/cancers14030498. Cancers (Basel). 2022. PMID: 35158766 Free PMC article. Review.
-
The formyl peptide receptor agonist FPRa14 induces differentiation of Neuro2a mouse neuroblastoma cells into multiple distinct morphologies which can be specifically inhibited with FPR antagonists and FPR knockdown using siRNA.PLoS One. 2019 Jun 6;14(6):e0217815. doi: 10.1371/journal.pone.0217815. eCollection 2019. PLoS One. 2019. PMID: 31170199 Free PMC article.
-
AaTs-1: A Tetrapeptide from Androctonus australis Scorpion Venom, Inhibiting U87 Glioblastoma Cells Proliferation by p53 and FPRL-1 Up-Regulations.Molecules. 2021 Dec 15;26(24):7610. doi: 10.3390/molecules26247610. Molecules. 2021. PMID: 34946686 Free PMC article.
-
Aurantiamide-related dipeptide derivatives are formyl peptide receptor 1 antagonists.Medchemcomm. 2019 Oct 7;10(12):2078-2088. doi: 10.1039/c9md00336c. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32206242 Free PMC article.
-
From odor to oncology: non-canonical odorant receptors in cancer.Oncogene. 2024 Jan;43(5):304-318. doi: 10.1038/s41388-023-02908-y. Epub 2023 Dec 12. Oncogene. 2024. PMID: 38087050 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

