Profilin 1 mutants form aggregates that induce accumulation of prion-like TDP-43
- PMID: 27432186
- PMCID: PMC5082967
- DOI: 10.1080/19336896.2016.1207033
Profilin 1 mutants form aggregates that induce accumulation of prion-like TDP-43
Abstract
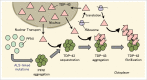
Mutations in the profilin 1 (PFN1) gene have been identified as a cause of familial amyotrophic lateral sclerosis (ALS), and neuropathological studies indicate that TDP-43 is accumulated in brains of patients with PFN1 mutation. Here, we investigated the role of PFN1 mutations in the formation of prion-like abnormal TDP-43. Expression of PFN1 with pathogenic mutations resulted in the formation of cytoplasmic aggregates positive for p62 and ubiquitin, and these aggregates sequestered endogenous TDP-43. TDP-43 accumulation was facilitated in the presence of proteasome or lysosome inhibitor. Co-expression of mutant PFN1 and TDP-43 increased the levels of detergent-insoluble and phosphorylated TDP-43, and this increase required the C-terminal region of TDP-43. Moreover, detergent-insoluble fractions prepared from cells expressing ALS-linked mutant PFN1 induced seed-dependent accumulation of TDP-43. These findings indicate that expression of PFN1 mutants induces accumulation of TDP-43, and promotes conversion of normal TDP-43 into an abnormal form. These results provide new insight into the mechanisms of TDP-43 proteinopathies and other diseases associated with amyloid-like protein deposition.
Keywords: ALS; FTLD; TDP-43; degradation system; prion; profilin 1.
Figures
Comment on
- Extra View to: Ratti A, Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem 2016; Epub ahead of print; PMID:; http://dx.doi.org/10.1111/jnc.13625 doi: 10.1111/jnc.13625
References
-
- Ayala YM, De Conti L, Avendano-Vazquez SE, Dhir A, Romano M, D'Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, et al.. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 2011; 30:277-88; PMID:21131904; http://dx.doi.org/10.1038/emboj.2010.310 - DOI - PMC - PubMed
-
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci 2008; 121:3778-85; PMID:18957508; http://dx.doi.org/10.1242/jcs.038950 - DOI - PubMed
-
- Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci 2008; 13:867-78; PMID:17981595; http://dx.doi.org/10.2741/2727 - DOI - PubMed
-
- Wang IF, Reddy NM, Shen CK. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci U S A 2002; 99:13583-8; PMID:12361981; http://dx.doi.org/10.1073/pnas.212483099 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
