Impaired Memory in OT-II Transgenic Mice Is Associated with Decreased Adult Hippocampal Neurogenesis Possibly Induced by Alteration in Th2 Cytokine Levels
- PMID: 27432189
- PMCID: PMC4990752
- DOI: 10.14348/molcells.2016.0072
Impaired Memory in OT-II Transgenic Mice Is Associated with Decreased Adult Hippocampal Neurogenesis Possibly Induced by Alteration in Th2 Cytokine Levels
Abstract
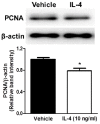
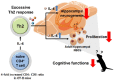
Recently, an increasing number of studies have focused on the effects of CD4+ T cell on cognitive function. However, the changes of Th2 cytokines in restricted CD4+ T cell receptor (TCR) repertoire model and their effects on the adult hippocampal neurogenesis and memory are not fully understood. Here, we investigated whether and how the mice with restricted CD4+ repertoire TCR exhibit learning and memory impairment by using OT-II mice. OT-II mice showed decreased adult neurogenesis in hippocampus and short- and long- term memory impairment. Moreover, Th2 cytokines in OT-II mice are significantly increased in peripheral organs and IL-4 is significantly increased in brain. Finally, IL-4 treatment significantly inhibited the proliferation of cultured adult rat hippocampal neural stem cells. Taken together, abnormal level of Th2 cytokines can lead memory dysfunction via impaired adult neurogenesis in OT-II transgenic.
Keywords: CD4 T cells; OT-II transgenic mice; Th2 cytokines; adult neurogenesis; cognition.
Figures


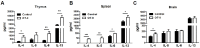

Comment in
-
Restricted CD4+ T cell receptor repertoire impairs cognitive function via alteration of Th2 cytokine levels.Neurogenesis (Austin). 2017 Jan 5;4(1):e1256856. doi: 10.1080/23262133.2016.1256856. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28229084 Free PMC article.
Similar articles
-
Restricted CD4+ T cell receptor repertoire impairs cognitive function via alteration of Th2 cytokine levels.Neurogenesis (Austin). 2017 Jan 5;4(1):e1256856. doi: 10.1080/23262133.2016.1256856. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28229084 Free PMC article.
-
Remodeling the Th1 polarized systemic environment contributes to neurogenesis and cognitive function via the Wnt7a pathway in neonatal mice.Neurobiol Learn Mem. 2017 May;141:60-71. doi: 10.1016/j.nlm.2017.03.002. Epub 2017 Mar 22. Neurobiol Learn Mem. 2017. PMID: 28342972
-
Nox4 Promotes Neural Stem/Precursor Cell Proliferation and Neurogenesis in the Hippocampus and Restores Memory Function Following Trimethyltin-Induced Injury.Neuroscience. 2019 Feb 1;398:193-205. doi: 10.1016/j.neuroscience.2018.11.046. Epub 2018 Dec 5. Neuroscience. 2019. PMID: 30528855
-
Evidence for the contribution of adult neurogenesis and hippocampal cell death in experimental cerebral malaria cognitive outcome.Neuroscience. 2015 Jan 22;284:920-933. doi: 10.1016/j.neuroscience.2014.10.062. Epub 2014 Nov 13. Neuroscience. 2015. PMID: 25451296
-
IL-4 in the brain: a cytokine to remember.J Immunol. 2012 Nov 1;189(9):4213-9. doi: 10.4049/jimmunol.1202246. J Immunol. 2012. PMID: 23087426 Free PMC article. Review.
Cited by
-
Immune conversations at the border: meningeal immunity in health and disease.Front Immunol. 2025 Jan 29;16:1531068. doi: 10.3389/fimmu.2025.1531068. eCollection 2025. Front Immunol. 2025. PMID: 39944687 Free PMC article. Review.
-
Advances in Meningeal Immunity.Trends Mol Med. 2018 Jun;24(6):542-559. doi: 10.1016/j.molmed.2018.04.003. Epub 2018 May 3. Trends Mol Med. 2018. PMID: 29731353 Free PMC article. Review.
-
Substrate-specific recognition of IKKs mediated by USP16 facilitates autoimmune inflammation.Sci Adv. 2021 Jan 13;7(3):eabc4009. doi: 10.1126/sciadv.abc4009. Print 2021 Jan. Sci Adv. 2021. PMID: 33523871 Free PMC article.
-
Neuroimmunology of depression.Adv Pharmacol. 2021;91:259-292. doi: 10.1016/bs.apha.2021.03.004. Epub 2021 Apr 26. Adv Pharmacol. 2021. PMID: 34099111 Free PMC article.
-
Restricted CD4+ T cell receptor repertoire impairs cognitive function via alteration of Th2 cytokine levels.Neurogenesis (Austin). 2017 Jan 5;4(1):e1256856. doi: 10.1080/23262133.2016.1256856. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28229084 Free PMC article.
References
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bannerman D.M., Sprengel R., Sanderson D.J., McHugh S.B., Rawlins J.N., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014;15:181–192. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

