Regulation of mitotic spindle orientation: an integrated view
- PMID: 27432284
- PMCID: PMC4967962
- DOI: 10.15252/embr.201642292
Regulation of mitotic spindle orientation: an integrated view
Abstract
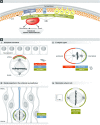
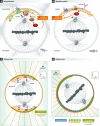
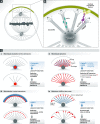
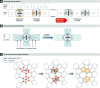
Mitotic spindle orientation is essential for cell fate decisions, epithelial maintenance, and tissue morphogenesis. In most animal cell types, the dynein motor complex is anchored at the cell cortex and exerts pulling forces on astral microtubules to position the spindle. Early studies identified the evolutionarily conserved Gαi/LGN/NuMA complex as a key regulator that polarizes cortical force generators. In recent years, a combination of genetics, biochemistry, modeling, and live imaging has contributed to decipher the mechanisms of spindle orientation. Here, we highlight the dynamic nature of the assembly of this complex and discuss the molecular regulation of its localization. Remarkably, a number of LGN-independent mechanisms were described recently, whereas NuMA remains central in most pathways involved in recruiting force generators at the cell cortex. We also describe the emerging role of the actin cortex in spindle orientation and discuss how dynamic astral microtubule formation is involved. We further give an overview on instructive external signals that control spindle orientation in tissues. Finally, we discuss the influence of cell geometry and mechanical forces on spindle orientation.
Keywords: NuMA; actin cortex; astral microtubules; cell geometry; spindle orientation.
© 2016 The Authors.
Figures


Similar articles
-
Evidence for dynein and astral microtubule-mediated cortical release and transport of Gαi/LGN/NuMA complex in mitotic cells.Mol Biol Cell. 2013 Apr;24(7):901-13. doi: 10.1091/mbc.E12-06-0458. Epub 2013 Feb 6. Mol Biol Cell. 2013. PMID: 23389635 Free PMC article.
-
Activated ezrin controls MISP levels to ensure correct NuMA polarization and spindle orientation.J Cell Sci. 2018 May 21;131(10):jcs214544. doi: 10.1242/jcs.214544. J Cell Sci. 2018. PMID: 29669740
-
The Aurora-A/TPX2 Axis Directs Spindle Orientation in Adherent Human Cells by Regulating NuMA and Microtubule Stability.Curr Biol. 2021 Feb 8;31(3):658-667.e5. doi: 10.1016/j.cub.2020.10.096. Epub 2020 Dec 3. Curr Biol. 2021. PMID: 33275894
-
Mechanisms of spindle positioning: cortical force generators in the limelight.Curr Opin Cell Biol. 2013 Dec;25(6):741-8. doi: 10.1016/j.ceb.2013.07.008. Epub 2013 Aug 16. Curr Opin Cell Biol. 2013. PMID: 23958212 Review.
-
Spindle positioning and its impact on vertebrate tissue architecture and cell fate.Nat Rev Mol Cell Biol. 2021 Oct;22(10):691-708. doi: 10.1038/s41580-021-00384-4. Epub 2021 Jun 22. Nat Rev Mol Cell Biol. 2021. PMID: 34158639 Free PMC article. Review.
Cited by
-
Understanding the underlying mechanisms governing spindle orientation: How far are we from there?J Cell Mol Med. 2022 Oct;26(19):4904-4910. doi: 10.1111/jcmm.17526. Epub 2022 Aug 27. J Cell Mol Med. 2022. PMID: 36029193 Free PMC article. Review.
-
Diversity of activator of G-protein signaling (AGS)-family proteins and their impact on asymmetric cell division across taxa.Dev Biol. 2020 Sep 15;465(2):89-99. doi: 10.1016/j.ydbio.2020.07.004. Epub 2020 Jul 18. Dev Biol. 2020. PMID: 32687894 Free PMC article. Review.
-
Shear-induced damped oscillations in an epithelium depend on actomyosin contraction and E-cadherin cell adhesion.Elife. 2018 Nov 14;7:e39640. doi: 10.7554/eLife.39640. Elife. 2018. PMID: 30427775 Free PMC article.
-
Combinatorial Contact Cues Specify Cell Division Orientation by Directing Cortical Myosin Flows.Dev Cell. 2018 Aug 6;46(3):257-270.e5. doi: 10.1016/j.devcel.2018.06.020. Epub 2018 Jul 19. Dev Cell. 2018. PMID: 30032990 Free PMC article.
-
Importin α Partitioning to the Plasma Membrane Regulates Intracellular Scaling.Cell. 2019 Feb 7;176(4):805-815.e8. doi: 10.1016/j.cell.2018.12.001. Epub 2019 Jan 10. Cell. 2019. PMID: 30639102 Free PMC article.
References
-
- Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA (1996) Role of inscuteable in orienting asymmetric cell divisions in Drosophila . Nature 383: 50–55 - PubMed
-
- Kemphues KJ, Priess JR, Morton DG, Cheng NS (1988) Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52: 311–320 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

