Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome
- PMID: 27432357
- PMCID: PMC5233467
- DOI: 10.1136/annrheumdis-2016-209442
Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome
Abstract
Objectives: Patients with antiphospholipid syndrome (APS) are at risk for subclinical endothelial injury, as well as accelerated atherosclerosis. In the related disease systemic lupus erythematosus, there is a well-established defect in circulating endothelial progenitors, which leads to an accrual of endothelial damage over time. This defect has been at least partially attributed to exaggerated expression of type I interferons (IFNs). We sought to determine whether these pathways are important in APS.
Methods: We studied 68 patients with primary APS. Endothelial progenitors were assessed by flow cytometry and functional assay. Type I IFN activity was determined by a well-accepted bioassay, while peripheral blood mononuclear cells were scored for expression of IFN-responsive genes.
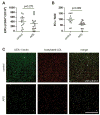
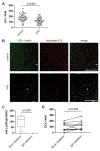
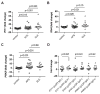
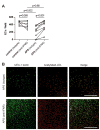
Results: Endothelial progenitors from patients with APS demonstrated a marked defect in the ability to differentiate into endothelial cells, a phenotype which could be mimicked by treating control progenitors with APS sera. Elevated type I IFN activity was detected in the circulation of patients with APS (a finding that was then replicated in an independent cohort). While IgG depletion from APS sera did not rescue endothelial progenitor function, the dysfunction was successfully reversed by a type I IFN receptor-neutralising antibody.
Conclusions: We describe, for the first time to our knowledge, an IFN signature in primary APS and show that this promotes impaired endothelial progenitor function. This work opens the door to novel approaches that may mitigate vascular damage in APS, such as anti-IFN drugs.
Keywords: Antiphospholipid Syndrome; Atherosclerosis; Cytokines.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
The authors have no competing interests or conflicts to disclose. AUTHORSHIP AND CONFLICT OF INTEREST DISCLOSURES The authors have no competing interests or conflicts to disclose. R.C.G., S.Y., A.A.G., N.M.K., C.N.-A., and D.H.-R. conducted experiments and analyzed data. A.R.C., W.J.M., P.L.B., and J.S.K. analyzed data and designed the study. All authors participated in writing the manuscript, and gave approval before submission.
Figures

Comment in
-
Monocyte type I interferon signature in antiphospholipid syndrome is related to proinflammatory monocyte subsets, hydroxychloroquine and statin use.Ann Rheum Dis. 2016 Dec;75(12):e81. doi: 10.1136/annrheumdis-2016-210485. Epub 2016 Sep 28. Ann Rheum Dis. 2016. PMID: 27689737 No abstract available.
-
Response to: 'Monocyte type I interferon signature in antiphospholipid syndrome is related to pro-inflammatory monocyte subsets, hydroxychloroquine and statin use' by van den Hoogen et al.Ann Rheum Dis. 2016 Dec;75(12):e82. doi: 10.1136/annrheumdis-2016-210529. Epub 2016 Oct 21. Ann Rheum Dis. 2016. PMID: 27769960 Free PMC article. No abstract available.
References
-
- Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arteriosclerosis, thrombosis, and vascular biology. 2006 Sep;26(9):2140–2146. - PubMed
-
- Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005 Jan 18;111(2):204–211. - PubMed
-
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003 Mar 4;107(8):1164–1169. - PubMed
-
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001 Feb 6;103(5):634–637. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

