Evaluation of Pharmacodynamic Interactions Between Telavancin and Aztreonam or Piperacillin/Tazobactam Against Pseudomonas aeruginosa, Escherichia coli and Methicillin-Resistant Staphylococcus aureus
- PMID: 27432414
- PMCID: PMC5019977
- DOI: 10.1007/s40121-016-0121-2
Evaluation of Pharmacodynamic Interactions Between Telavancin and Aztreonam or Piperacillin/Tazobactam Against Pseudomonas aeruginosa, Escherichia coli and Methicillin-Resistant Staphylococcus aureus
Abstract
Introduction: In clinical trials comparing telavancin (TLV) with vancomycin for treatment of hospital-acquired pneumonia, TLV demonstrated lower clinical cure rates than vancomycin in patients who had mixed gram-positive and -negative infections and were concomitantly treated with either aztreonam (ATM) or piperacillin/tazobactam (PTZ). Here, we investigated therapeutic interactions between TLV and ATM or PTZ in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model under simulated reduced renal function conditions.
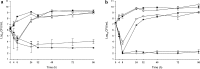
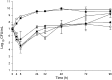
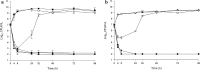
Methods: In vitro one-compartment PK/PD models were run over 96 h simulating TLV 10 mg/kg every 48 h, ATM 500 mg every 8 h and PTZ continuous infusion 13.5 g over 24 h alone and in combination against P. aeruginosa, E. coli and methicillin-resistant S. aureus (MRSA). The efficacy of antimicrobials was evaluated by plotting time-kill curves and calculating the reduction in log10 cfu/ml over 96 h.
Results: Against both MRSA strains, TLV was rapidly bactericidal at 4 h and maintained its activity over 96 h with no observed antagonism by either ATM or PTZ. PTZ maintained bacteriostatic and bactericidal activities against E. coli ATCC 25922 and clinical strain R1022 at 96 h, whereas both strains regrew as soon as 24 h in ATM models. Against P. aeruginosa ATCC 27853, regrowth was noted at 24 h in models simulating ATM and PTZ. The addition of TLV to ATM or PTZ had no appreciable impact on activity against the two E. coli strains and P. aeruginosa strain.
Conclusions: The combinations of TLV and either ATM or PTZ did not demonstrate any antagonistic activity. Clinical variables and patient characteristics should be further explored to determine possible reasons for discrepancies in outcomes.
Funding: Theravance Biopharma Antibiotics, Inc.
Keywords: Aztreonam; Drug interactions; Escherichia coli; Methicillin resistant; Piperacillin/tazobactam; Pseudomonas aeruginosa; Staphylococcus aureus; Telavancin.
Figures
Similar articles
-
Evaluation of Telavancin Alone and Combined with Ceftaroline or Rifampin against Methicillin-Resistant Staphylococcus aureus in an In Vitro Biofilm Model.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00567-18. doi: 10.1128/AAC.00567-18. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29784849 Free PMC article.
-
Vancomycin and piperacillin-tazobactam against methicillin-resistant Staphylococcus aureus and vancomycin-intermediate Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model.Clin Ther. 2014 Oct 1;36(10):1334-44. doi: 10.1016/j.clinthera.2014.06.027. Epub 2014 Jul 25. Clin Ther. 2014. PMID: 25066667
-
Telavancin demonstrates activity against methicillin-resistant Staphylococcus aureus isolates with reduced susceptibility to vancomycin, daptomycin, and linezolid in broth microdilution MIC and one-compartment pharmacokinetic/pharmacodynamic models.Antimicrob Agents Chemother. 2015 Sep;59(9):5529-34. doi: 10.1128/AAC.00773-15. Epub 2015 Jun 29. Antimicrob Agents Chemother. 2015. PMID: 26124162 Free PMC article.
-
Telavancin in the treatment of Staphylococcus aureus hospital-acquired and ventilator-associated pneumonia: clinical evidence and experience.Ther Adv Respir Dis. 2016 Aug;10(4):368-78. doi: 10.1177/1753465816651594. Epub 2016 Jun 23. Ther Adv Respir Dis. 2016. PMID: 27340253 Free PMC article. Review.
-
Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin.Am J Clin Dermatol. 2008;9(4):245-54. doi: 10.2165/00128071-200809040-00004. Am J Clin Dermatol. 2008. PMID: 18572975 Review.
Cited by
-
Evaluation of Telavancin Alone and Combined with Ceftaroline or Rifampin against Methicillin-Resistant Staphylococcus aureus in an In Vitro Biofilm Model.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00567-18. doi: 10.1128/AAC.00567-18. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29784849 Free PMC article.
-
Clinical Pharmacokinetics and Pharmacodynamics of Telavancin Compared with the Other Glycopeptides.Clin Pharmacokinet. 2018 Jul;57(7):797-816. doi: 10.1007/s40262-017-0623-4. Clin Pharmacokinet. 2018. PMID: 29332251 Free PMC article. Review.
References
-
- Theravance Biopharma Antibiotics Inc. VIBATIV (Telavancin) For injection, US Prescribing Information. 2016. https://www.vibativ.com/pdf/PrescribingInformation.pdf. Accessed 13 July 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

