Effect of modified live or inactivated feline herpesvirus-1 parenteral vaccines on clinical and laboratory findings following viral challenge
- PMID: 27432436
- PMCID: PMC11104124
- DOI: 10.1177/1098612X16659333
Effect of modified live or inactivated feline herpesvirus-1 parenteral vaccines on clinical and laboratory findings following viral challenge
Abstract
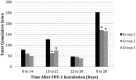
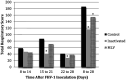
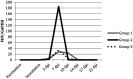
Objectives The objective was to investigate the effect of one dose of an inactivated feline herpesvirus-1 (FHV-1), feline calicivirus (FCV) and panleukopenia virus (FPV) vaccine (FVRCP) or one dose of a modified live (ML) FVRCP vaccine on clinical signs and shedding of FHV-1 in specific pathogen-free kittens after challenge with FHV-1 7 days after vaccination. Methods Twenty-four FHV-1 seronegative 5-month-old kittens were randomized into three groups of eight kittens. Group 1 kittens were maintained as unvaccinated controls, group 2 kittens were administered one dose of the inactivated FVRCP vaccine subcutaneously (SC) and group 3 kittens were administered one dose of the ML FVRCP vaccine SC. All 24 cats were administered FHV-1 by nasal and oropharyngeal inoculation 7 days later and were observed daily for clinical signs of illness for 21 days. Results In the 21 days after FHV-1 challenge, both groups of vaccinated cats were less likely to be clinically ill (indicated by lower cumulative clinical scores) than control cats ( P <0.001). There was no statistical difference in total clinical score between the two vaccinated groups ( P = 0.97). Although the total clinical score was similar between both vaccines, signs of respiratory disease were significantly fewer in the kittens vaccinated with the inactivated FVRCP vaccine compared with the ML FVRCP vaccine ( P = 0.005) during the period after inoculation when the majority of clinical disease was observed. Conclusions and relevance Parenteral administration of either the inactivated FVRCP vaccine or the ML FVRCP vaccine can decrease clinical signs of illness due to FHV-1 on a day 7 challenge when compared with controls. Use of either vaccine product is indicated in cats at risk of acute exposure to FHV-1.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Feline panleukopenia virus, feline herpesvirus-1 and feline calicivirus antibody responses in seronegative specific pathogen-free kittens after parenteral administration of an inactivated FVRCP vaccine or a modified live FVRCP vaccine.J Feline Med Surg. 2012 Feb;14(2):161-4. doi: 10.1177/1098612X11432240. J Feline Med Surg. 2012. PMID: 22314095 Free PMC article.
-
Feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus antibody responses in seronegative specific pathogen-free cats after a single administration of two different modified live FVRCP vaccines.J Feline Med Surg. 2009 Feb;11(2):159-62. doi: 10.1016/j.jfms.2008.05.004. Epub 2008 Sep 7. J Feline Med Surg. 2009. PMID: 18782676 Free PMC article.
-
Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus.Vet J. 2009 Oct;182(1):86-93. doi: 10.1016/j.tvjl.2008.05.025. Epub 2008 Aug 9. Vet J. 2009. PMID: 18694649 Clinical Trial.
-
Feline herpesvirus infection. ABCD guidelines on prevention and management.J Feline Med Surg. 2009 Jul;11(7):547-55. doi: 10.1016/j.jfms.2009.05.003. J Feline Med Surg. 2009. PMID: 19481034 Free PMC article. Review.
-
Feline herpesvirus.Clin Tech Small Anim Pract. 2003 Aug;18(3):178-85. doi: 10.1016/s1096-2867(03)90014-4. Clin Tech Small Anim Pract. 2003. PMID: 14604092 Review.
Cited by
-
Effect of a Pheromone on Stress-Associated Reactivation of Feline Herpesvirus-1 in Experimentally Inoculated Kittens.J Vet Intern Med. 2018 Jan;32(1):406-417. doi: 10.1111/jvim.14894. Epub 2017 Dec 8. J Vet Intern Med. 2018. PMID: 29219213 Free PMC article. Clinical Trial.
-
Cellular and humoral immune responses in cats vaccinated with feline herpesvirus 1 modified live virus vaccine.Front Vet Sci. 2025 Jan 15;11:1516850. doi: 10.3389/fvets.2024.1516850. eCollection 2024. Front Vet Sci. 2025. PMID: 39881722 Free PMC article.
-
Novel Strain-Based Triple Inactivated Vaccine Confers Rapid Neutralizing Immunity to Feline Multisystemic Pathogens With Two-Dose Regimen.Transbound Emerg Dis. 2025 Aug 7;2025:9642624. doi: 10.1155/tbed/9642624. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40822450 Free PMC article.
-
Evaluation of liposome toll-like receptor ligand complexes for non-specific mucosal immunoprotection from feline herpesvirus-1 infection.J Vet Intern Med. 2019 Mar;33(2):831-837. doi: 10.1111/jvim.15427. Epub 2019 Mar 7. J Vet Intern Med. 2019. PMID: 30847973 Free PMC article.
-
Antibody response to feline herpesvirus-1 vaccination in healthy adult cats.J Feline Med Surg. 2020 Apr;22(4):329-338. doi: 10.1177/1098612X19845702. Epub 2019 May 13. J Feline Med Surg. 2020. PMID: 31079527 Free PMC article.
References
-
- Veir J, Ruch-Gallie R, Spindel ME, et al.. Prevalence of FHV-1, Mycoplasma spp., and aerobic bacteria in shelter kittens with acute upper respiratory tract disease. J Vet Intern Med 2004; 18: 375–460.
-
- Fontenelle JP, Powell CC, Veir JK, et al.. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesviurs-1 infection in cats. Am J Vet Res 2008; 69: 289–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

